Difference between revisions of "MutS"
| Line 80: | Line 80: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| Line 100: | Line 100: | ||
* '''Structure:''' | * '''Structure:''' | ||
| + | ** [http://www.pdb.org/pdb/explore/explore.do?structureId=1ng9 1NG9] (from ''E. coli'', 39% identity) {{PubMed|12554674}} | ||
* '''UniProt:''' [http://www.uniprot.org/uniprot/P49849 P49849] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P49849 P49849] | ||
| Line 149: | Line 150: | ||
<pubmed> 22933559 </pubmed> | <pubmed> 22933559 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed>8760914, 15375129,16479537 20525796 20453097 23228104 21958350 23882084 23998896 24062730 </pubmed> | + | <pubmed>8760914, 15375129,16479537 20525796 20453097 23228104 21958350 23882084 23998896 24062730 12554674 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:24, 27 February 2014
- Description: DNA mismatch repair (mismatch recognition protein)
| Gene name | mutS |
| Synonyms | |
| Essential | no |
| Product | mismatch recognition protein |
| Function | DNA repair |
| Gene expression levels in SubtiExpress: mutS | |
| Interactions involving this protein in SubtInteract: MutS | |
| MW, pI | 97 kDa, 5.189 |
| Gene length, protein length | 2574 bp, 858 aa |
| Immediate neighbours | cotE, mutL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed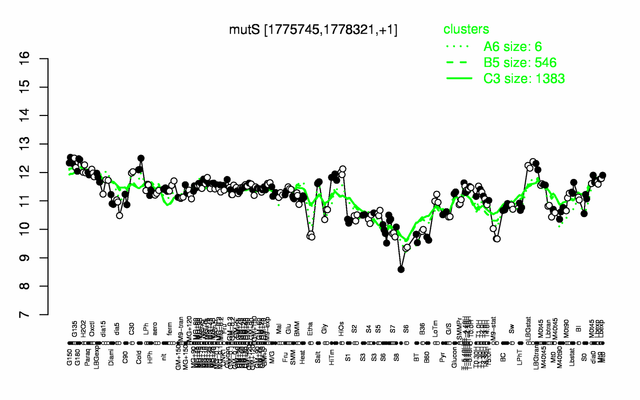
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU17040
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- MutS recognizes mismatches in the DNA and recruits MutL to the site of mismatch recognition
- Protein family: DNA mismatch repair mutS family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- UniProt: P49849
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- An antisense RNA is predicted for mutS PubMed
- intracellular concentration: about 80 dimers (100 nM) PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Justin S Lenhart, Jeremy W Schroeder, Brian W Walsh, Lyle A Simmons
DNA repair and genome maintenance in Bacillus subtilis.
Microbiol Mol Biol Rev: 2012, 76(3);530-64
[PubMed:22933559]
[WorldCat.org]
[DOI]
(I p)
Original publications
Motohiro Akashi, Hirofumi Yoshikawa
Relevance of GC content to the conservation of DNA polymerase III/mismatch repair system in Gram-positive bacteria.
Front Microbiol: 2013, 4;266
[PubMed:24062730]
[WorldCat.org]
[DOI]
(P e)
Justin S Lenhart, Monica C Pillon, Alba Guarné, Lyle A Simmons
Trapping and visualizing intermediate steps in the mismatch repair pathway in vivo.
Mol Microbiol: 2013, 90(4);680-98
[PubMed:23998896]
[WorldCat.org]
[DOI]
(I p)
Nina Y Yao, Jeremy W Schroeder, Olga Yurieva, Lyle A Simmons, Mike E O'Donnell
Cost of rNTP/dNTP pool imbalance at the replication fork.
Proc Natl Acad Sci U S A: 2013, 110(32);12942-7
[PubMed:23882084]
[WorldCat.org]
[DOI]
(I p)
Justin S Lenhart, Anushi Sharma, Manju M Hingorani, Lyle A Simmons
DnaN clamp zones provide a platform for spatiotemporal coupling of mismatch detection to DNA replication.
Mol Microbiol: 2013, 87(3);553-68
[PubMed:23228104]
[WorldCat.org]
[DOI]
(I p)
Andrew D Klocko, Jeremy W Schroeder, Brian W Walsh, Justin S Lenhart, Margery L Evans, Lyle A Simmons
Mismatch repair causes the dynamic release of an essential DNA polymerase from the replication fork.
Mol Microbiol: 2011, 82(3);648-63
[PubMed:21958350]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Nicole M Dupes, Brian W Walsh, Andrew D Klocko, Justin S Lenhart, Heather L Peterson, David A Gessert, Cassie E Pavlick, Lyle A Simmons
Mutations in the Bacillus subtilis beta clamp that separate its roles in DNA replication from mismatch repair.
J Bacteriol: 2010, 192(13);3452-63
[PubMed:20453097]
[WorldCat.org]
[DOI]
(I p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Mario Pedraza-Reyes, Ronald E Yasbin
Contribution of the mismatch DNA repair system to the generation of stationary-phase-induced mutants of Bacillus subtilis.
J Bacteriol: 2004, 186(19);6485-91
[PubMed:15375129]
[WorldCat.org]
[DOI]
(P p)
Meindert H Lamers, Herrie H K Winterwerp, Titia K Sixma
The alternating ATPase domains of MutS control DNA mismatch repair.
EMBO J: 2003, 22(3);746-56
[PubMed:12554674]
[WorldCat.org]
[DOI]
(P p)
F Ginetti, M Perego, A M Albertini, A Galizzi
Bacillus subtilis mutS mutL operon: identification, nucleotide sequence and mutagenesis.
Microbiology (Reading): 1996, 142 ( Pt 8);2021-9
[PubMed:8760914]
[WorldCat.org]
[DOI]
(P p)