MurQ
- Description: similar to E. coli MurQ (etherase, cleaves lactate from N-acetylmuramic acid)
| Gene name | murQ |
| Synonyms | ybbI |
| Essential | no |
| Product | putative etherase |
| Function | cell wall turnover |
| Gene expression levels in SubtiExpress: murQ | |
| Metabolic function and regulation of this protein in SubtiPathways: murQ | |
| MW, pI | 32 kDa, 5.404 |
| Gene length, protein length | 912 bp, 304 aa |
| Immediate neighbours | murR, ybbJ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 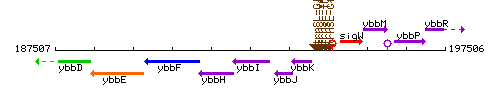
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed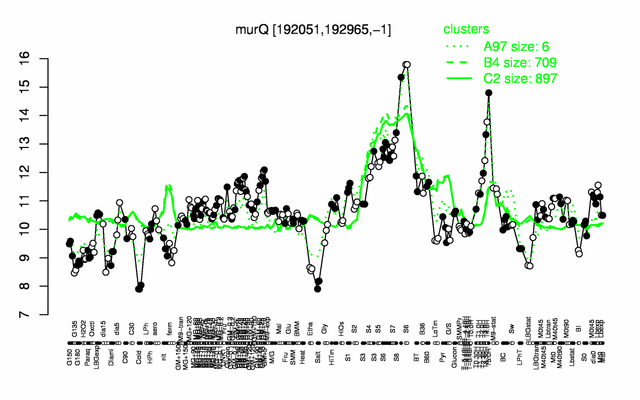
| |
Contents
Categories containing this gene/protein
cell wall degradation/ turnover, utilization of specific carbon sources, phosphoproteins, sporulation/ other
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01700
Phenotypes of a mutant
Database entries
- BsubCyc: BSU01700
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: N-acetylmuramic acid 6-phosphate + H2O = N-acetyl-D-glucosamine 6-phosphate + D-lactate (according to Swiss-Prot)
- Protein family: SIS domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Ser-2 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU01700
- Structure:
- UniProt: Q45582
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Silke Litzinger, Amanda Duckworth, Katja Nitzsche, Christian Risinger, Valentin Wittmann, Christoph Mayer
Muropeptide rescue in Bacillus subtilis involves sequential hydrolysis by beta-N-acetylglucosaminidase and N-acetylmuramyl-L-alanine amidase.
J Bacteriol: 2010, 192(12);3132-43
[PubMed:20400549]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)