Difference between revisions of "MtnD"
| Line 118: | Line 118: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ** belongs to the 100 [[most abundant proteins]] {{PubMed|15378759}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 4182 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 1417 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 09:24, 17 April 2014
- Description: 1,2,-dihydroxy-3-keto-5-methylthiopentene dioxygenase
| Gene name | mtnD |
| Synonyms | ykrZ |
| Essential | no |
| Product | 1,2,-dihydroxy-3-keto-5-methylthiopentene dioxygenase |
| Function | methionine salvage |
| Gene expression levels in SubtiExpress: mtnD | |
| Metabolic function and regulation of this protein in SubtiPathways: mtnD | |
| MW, pI | 20 kDa, 4.408 |
| Gene length, protein length | 534 bp, 178 aa |
| Immediate neighbours | mtnB, ykvA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 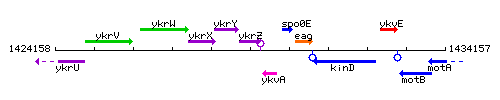
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed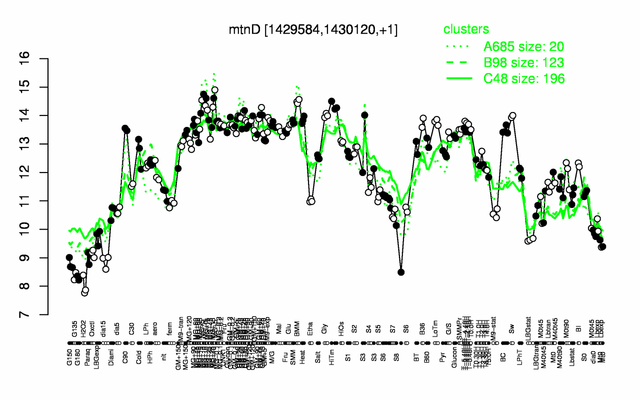
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU13620
Phenotypes of a mutant
Database entries
- BsubCyc: BSU13620
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 1,2-dihydroxy-5-(methylthio)pent-1-en-3-one + O2 = 3-(methylthio)propanoate + formate + CO (according to Swiss-Prot)
- Protein family: acireductone dioxygenase (ARD) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU13620
- Structure:
- UniProt: O31669
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism: S-box: transcription termination/ antitermination, the S-box riboswitch binds S-adenosylmethionine resulting in termination PubMed
- Additional information:
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 4182 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 1417 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Jerneja Tomsic, Brooke A McDaniel, Frank J Grundy, Tina M Henkin
Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S-box elements in bacillus subtilis exhibit differential sensitivity to SAM In vivo and in vitro.
J Bacteriol: 2008, 190(3);823-33
[PubMed:18039762]
[WorldCat.org]
[DOI]
(I p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)
Agnieszka Sekowska, Valérie Dénervaud, Hiroki Ashida, Karine Michoud, Dieter Haas, Akiho Yokota, Antoine Danchin
Bacterial variations on the methionine salvage pathway.
BMC Microbiol: 2004, 4;9
[PubMed:15102328]
[WorldCat.org]
[DOI]
(I e)
Maumita Mandal, Benjamin Boese, Jeffrey E Barrick, Wade C Winkler, Ronald R Breaker
Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria.
Cell: 2003, 113(5);577-86
[PubMed:12787499]
[WorldCat.org]
[DOI]
(P p)
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)
Agnieszka Sekowska, Antoine Danchin
The methionine salvage pathway in Bacillus subtilis.
BMC Microbiol: 2002, 2;8
[PubMed:12022921]
[WorldCat.org]
[DOI]
(I e)
Brooke A Murphy, Frank J Grundy, Tina M Henkin
Prediction of gene function in methylthioadenosine recycling from regulatory signals.
J Bacteriol: 2002, 184(8);2314-8
[PubMed:11914366]
[WorldCat.org]
[DOI]
(P p)