Difference between revisions of "MrpA"
| Line 60: | Line 60: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU31600&redirect=T BSU31600] | ||
* '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/mrpABCDEFG.html] | * '''DBTBS entry:''' [http://dbtbs.hgc.jp/COG/prom/mrpABCDEFG.html] | ||
| Line 96: | Line 97: | ||
=== Database entries === | === Database entries === | ||
| + | * '''BsubCyc:''' [http://bsubcyc.org/BSUB/NEW-IMAGE?type=NIL&object=BSU31600&redirect=T BSU31600] | ||
* '''Structure:''' | * '''Structure:''' | ||
Revision as of 14:38, 2 April 2014
- Description: Na+ transporter subunit of the Na+/H+ antiporter, multiple resistance and pH homeostasis
| Gene name | mrpA |
| Synonyms | yufT, shaA, ntrA |
| Essential | yes PubMed |
| Product | Na+/H+ antiporter subunit |
| Function | sodium export |
| Gene expression levels in SubtiExpress: mrpA | |
| Interactions involving this protein in SubtInteract: MrpA | |
| MW, pI | 86 kDa, 9.519 |
| Gene length, protein length | 2322 bp, 774 aa |
| Immediate neighbours | yufS, mrpB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 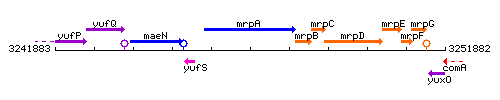
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed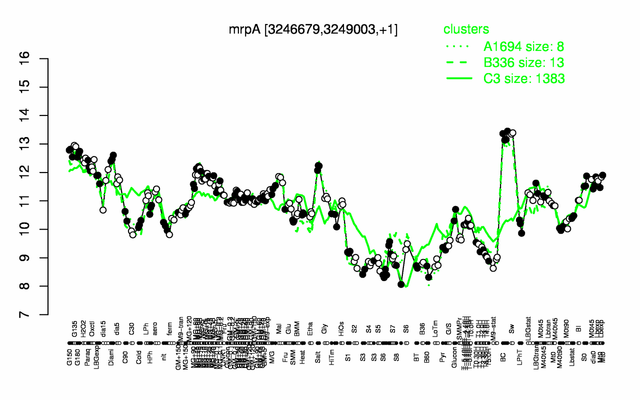
| |
Contents
Categories containing this gene/protein
transporters/ other, metal ion homeostasis (K, Na, Ca, Mg), essential genes, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU31600
Phenotypes of a mutant
- essential PubMed
- a mrpA mutant is viable at pH7.4 and sodium concentrations below 80 mM PubMed
- the mrpA mutant accumulates internal sodium PubMed
Database entries
- BsubCyc: BSU31600
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Na+ transporter subunit of the Na+/H+ antiporter PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU31600
- Structure:
- UniProt: Q9K2S2
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Kamil Górecki, Cecilia Hägerhäll, Torbjörn Drakenberg
The Na+ transport in gram-positive bacteria defect in the Mrp antiporter complex measured with 23Na nuclear magnetic resonance.
Anal Biochem: 2014, 445;80-6
[PubMed:24139955]
[WorldCat.org]
[DOI]
(I p)
Vamsi K Moparthi, Brijesh Kumar, Yusra Al-Eryani, Eva Sperling, Kamil Górecki, Torbjörn Drakenberg, Cecilia Hägerhäll
Functional role of the MrpA- and MrpD-homologous protein subunits in enzyme complexes evolutionary related to respiratory chain complex I.
Biochim Biophys Acta: 2014, 1837(1);178-85
[PubMed:24095649]
[WorldCat.org]
[DOI]
(P p)
Egle Virzintiene, Vamsi K Moparthi, Yusra Al-Eryani, Leonard Shumbe, Kamil Górecki, Cecilia Hägerhäll
Structure and function of the C-terminal domain of MrpA in the Bacillus subtilis Mrp-antiporter complex--the evolutionary progenitor of the long horizontal helix in complex I.
FEBS Lett: 2013, 587(20);3341-7
[PubMed:24021651]
[WorldCat.org]
[DOI]
(I p)
Vamsi K Moparthi, Brijesh Kumar, Cecilie Mathiesen, Cecilia Hägerhäll
Homologous protein subunits from Escherichia coli NADH:quinone oxidoreductase can functionally replace MrpA and MrpD in Bacillus subtilis.
Biochim Biophys Acta: 2011, 1807(4);427-36
[PubMed:21236240]
[WorldCat.org]
[DOI]
(P p)
Yusuke Kajiyama, Masato Otagiri, Junichi Sekiguchi, Toshiaki Kudo, Saori Kosono
The MrpA, MrpB and MrpD subunits of the Mrp antiporter complex in Bacillus subtilis contain membrane-embedded and essential acidic residues.
Microbiology (Reading): 2009, 155(Pt 7);2137-2147
[PubMed:19389778]
[WorldCat.org]
[DOI]
(P p)
Yusuke Kajiyama, Masato Otagiri, Junichi Sekiguchi, Saori Kosono, Toshiaki Kudo
Complex formation by the mrpABCDEFG gene products, which constitute a principal Na+/H+ antiporter in Bacillus subtilis.
J Bacteriol: 2007, 189(20);7511-4
[PubMed:17693497]
[WorldCat.org]
[DOI]
(P p)
Saori Kosono, Yusuke Kajiyama, Shin Kawasaki, Toko Yoshinaka, Koki Haga, Toshiaki Kudo
Functional involvement of membrane-embedded and conserved acidic residues in the ShaA subunit of the multigene-encoded Na+/H+ antiporter in Bacillus subtilis.
Biochim Biophys Acta: 2006, 1758(5);627-35
[PubMed:16730649]
[WorldCat.org]
[DOI]
(P p)
Saori Kosono, Kei Asai, Yoshito Sadaie, Toshiaki Kudo
Altered gene expression in the transition phase by disruption of a Na+/H+ antiporter gene (shaA) in Bacillus subtilis.
FEMS Microbiol Lett: 2004, 232(1);93-9
[PubMed:15019740]
[WorldCat.org]
[DOI]
(P p)
M Ito, A A Guffanti, W Wang, T A Krulwich
Effects of nonpolar mutations in each of the seven Bacillus subtilis mrp genes suggest complex interactions among the gene products in support of Na(+) and alkali but not cholate resistance.
J Bacteriol: 2000, 182(20);5663-70
[PubMed:11004162]
[WorldCat.org]
[DOI]
(P p)
M Ito, A A Guffanti, B Oudega, T A Krulwich
mrp, a multigene, multifunctional locus in Bacillus subtilis with roles in resistance to cholate and to Na+ and in pH homeostasis.
J Bacteriol: 1999, 181(8);2394-402
[PubMed:10198001]
[WorldCat.org]
[DOI]
(P p)