Difference between revisions of "MraY"
(→References) |
|||
| Line 133: | Line 133: | ||
* '''two-hybrid system:''' | * '''two-hybrid system:''' | ||
| + | |||
| + | * '''FLAG-tag construct:''' | ||
| + | ** GP2001 ''mraY-3xFLAG ermC'' (based on [[pGP1087]]), available in [[Jörg Stülke]]'s lab | ||
* '''Antibody:''' | * '''Antibody:''' | ||
Revision as of 16:53, 15 August 2014
- Description: phospho-N-acetylmuramoyl-pentapeptide-transferase (meso-2,6-diaminopimelate), catalyzes the first commited membrane-bound step of bacterial peptidoglycan synthesis leading to the formation of lipid I
| Gene name | mraY |
| Synonyms | |
| Essential | yes PubMed |
| Product | phospho-N-acetylmuramoyl-pentapeptide-transferase (meso-2,6-diaminopimelate) |
| Function | peptidoglycan precursor biosynthesis |
| Gene expression levels in SubtiExpress: mraY | |
| Metabolic function and regulation of this protein in SubtiPathways: mraY | |
| MW, pI | 35 kDa, 8.966 |
| Gene length, protein length | 972 bp, 324 aa |
| Immediate neighbours | murE, murD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 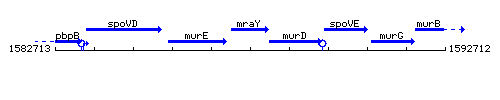
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed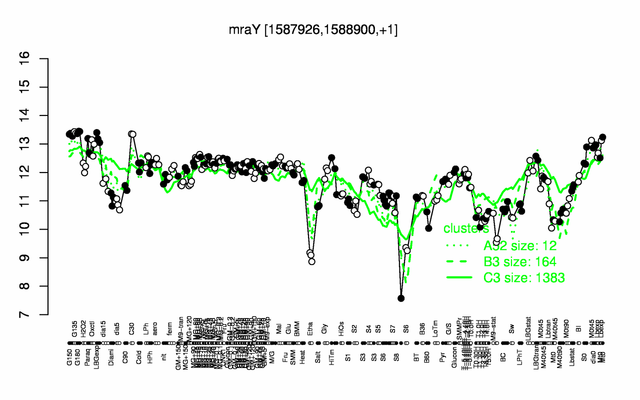
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, essential genes, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15190
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU15190
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: UDP-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala) + undecaprenyl phosphate = UMP + Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol (according to Swiss-Prot)
- Protein family: MraY subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU15190
- Structure:
- UniProt: Q03521
- KEGG entry: [3]
- E.C. number: 2.7.8.13
Additional information
Expression and regulation
- Regulation:
- expressed during vegatative growth PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- FLAG-tag construct:
- GP2001 mraY-3xFLAG ermC (based on pGP1087), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Maria T Rodolis, Agnes Mihalyi, Amy O'Reilly, Justinas Slikas, David I Roper, Robert E W Hancock, Timothy D H Bugg
Identification of a novel inhibition site in translocase MraY based upon the site of interaction with lysis protein E from bacteriophage ϕX174.
Chembiochem: 2014, 15(9);1300-8
[PubMed:24895118]
[WorldCat.org]
[DOI]
(I p)
Yi Ma, Daniela Münch, Tanja Schneider, Hans-Georg Sahl, Ahmed Bouhss, Umesh Ghoshdastider, Jufang Wang, Volker Dötsch, Xiaoning Wang, Frank Bernhard
Preparative scale cell-free production and quality optimization of MraY homologues in different expression modes.
J Biol Chem: 2011, 286(45);38844-53
[PubMed:21937437]
[WorldCat.org]
[DOI]
(I p)
Delphine Lecerclé, Anthony Clouet, Bayan Al-Dabbagh, Muriel Crouvoisier, Ahmed Bouhss, Christine Gravier-Pelletier, Yves Le Merrer
Bacterial transferase MraY inhibitors: synthesis and biological evaluation.
Bioorg Med Chem: 2010, 18(12);4560-9
[PubMed:20537545]
[WorldCat.org]
[DOI]
(I p)
Yi Zheng, Douglas K Struck, Thomas G Bernhardt, Ry Young
Genetic analysis of MraY inhibition by the phiX174 protein E.
Genetics: 2008, 180(3);1459-66
[PubMed:18791230]
[WorldCat.org]
[DOI]
(P p)
Bayan Al-Dabbagh, Xavier Henry, Meriem El Ghachi, Geneviève Auger, Didier Blanot, Claudine Parquet, Dominique Mengin-Lecreulx, Ahmed Bouhss
Active site mapping of MraY, a member of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily, catalyzing the first membrane step of peptidoglycan biosynthesis.
Biochemistry: 2008, 47(34);8919-28
[PubMed:18672909]
[WorldCat.org]
[DOI]
(I p)
Ahmed Bouhss, Amy E Trunkfield, Timothy D H Bugg, Dominique Mengin-Lecreulx
The biosynthesis of peptidoglycan lipid-linked intermediates.
FEMS Microbiol Rev: 2008, 32(2);208-33
[PubMed:18081839]
[WorldCat.org]
[DOI]
(P p)
Ahmed Bouhss, Muriel Crouvoisier, Didier Blanot, Dominique Mengin-Lecreulx
Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis.
J Biol Chem: 2004, 279(29);29974-80
[PubMed:15131133]
[WorldCat.org]
[DOI]
(P p)
A A Branstrom, S Midha, C B Longley, K Han, E R Baizman, H R Axelrod
Assay for identification of inhibitors for bacterial MraY translocase or MurG transferase.
Anal Biochem: 2000, 280(2);315-9
[PubMed:10790316]
[WorldCat.org]
[DOI]
(P p)
M A Lehrman
A family of UDP-GlcNAc/MurNAc: polyisoprenol-P GlcNAc/MurNAc-1-P transferases.
Glycobiology: 1994, 4(6);768-71
[PubMed:7734839]
[WorldCat.org]
[DOI]
(P p)
R A Daniel, J Errington
DNA sequence of the murE-murD region of Bacillus subtilis 168.
J Gen Microbiol: 1993, 139(2);361-70
[PubMed:8436954]
[WorldCat.org]
[DOI]
(P p)
A O Henriques, H de Lencastre, P J Piggot
A Bacillus subtilis morphogene cluster that includes spoVE is homologous to the mra region of Escherichia coli.
Biochimie: 1992, 74(7-8);735-48
[PubMed:1391053]
[WorldCat.org]
[DOI]
(P p)