Mpr
- Description: extracellular metalloprotease
| Gene name | mpr |
| Synonyms | |
| Essential | no |
| Product | extracellular metalloprotease |
| Function | protein degradation |
| Gene expression levels in SubtiExpress: mpr | |
| MW, pI | 33 kDa, 8.967 |
| Gene length, protein length | 939 bp, 313 aa |
| Immediate neighbours | purT, ybfJ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 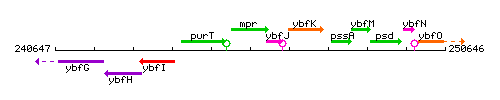
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed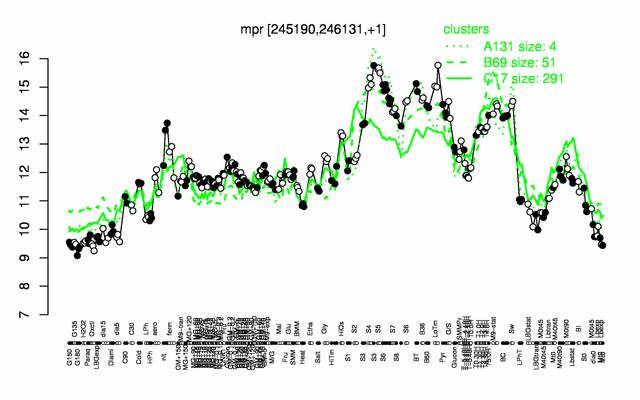
| |
Contents
Categories containing this gene/protein
utilization of nitrogen sources other than amino acids, proteolysis
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU02240
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase S1B family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization:
- extracellular (signal peptide) PubMed
Database entries
- Structure:
- UniProt: P39790
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Regulation: repressed by casamino acids PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications
Susanne Pohl, Gaurav Bhavsar, Joanne Hulme, Alexandra E Bloor, Goksel Misirli, Matthew W Leckenby, David S Radford, Wendy Smith, Anil Wipat, E Diane Williamson, Colin R Harwood, Rocky M Cranenburgh
Proteomic analysis of Bacillus subtilis strains engineered for improved production of heterologous proteins.
Proteomics: 2013, 13(22);3298-308
[PubMed:24115457]
[WorldCat.org]
[DOI]
(I p)
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Chi Hye Park, Sang Jun Lee, Sung Gu Lee, Weon Sup Lee, Si Myung Byun
Hetero- and autoprocessing of the extracellular metalloprotease (Mpr) in Bacillus subtilis.
J Bacteriol: 2004, 186(19);6457-64
[PubMed:15375126]
[WorldCat.org]
[DOI]
(P p)
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)