Difference between revisions of "MoaE"
(→Original publications) |
|||
| Line 137: | Line 137: | ||
<pubmed> 22616866 </pubmed> | <pubmed> 22616866 </pubmed> | ||
== Original publications == | == Original publications == | ||
| − | <pubmed> 12571227 </pubmed> | + | <pubmed> 12571227 22383849</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 14:37, 4 June 2012
- Description: molybdopterin synthase (large subunit), catalyses the transfer of sulfide from MoaD thiocarboxylate
| Gene name | moaE |
| Synonyms | |
| Essential | no |
| Product | molybdopterin synthase (large subunit) |
| Function | nitrate respiration |
| MW, pI | 17 kDa, 4.746 |
| Gene length, protein length | 471 bp, 157 aa |
| Immediate neighbours | mobB, moaD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 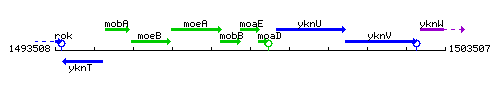
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14300
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: catalyses the transfer of sulfide from MoaD thiocarboxylate
- Protein family: moaE family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O31705
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Tadhg P Begley, Steven E Ealick, Fred W McLafferty
Thiamin biosynthesis: still yielding fascinating biological chemistry.
Biochem Soc Trans: 2012, 40(3);555-60
[PubMed:22616866]
[WorldCat.org]
[DOI]
(I p)
Original publications
Pierre Nicolas, Ulrike Mäder, Etienne Dervyn, Tatiana Rochat, Aurélie Leduc, Nathalie Pigeonneau, Elena Bidnenko, Elodie Marchadier, Mark Hoebeke, Stéphane Aymerich, Dörte Becher, Paola Bisicchia, Eric Botella, Olivier Delumeau, Geoff Doherty, Emma L Denham, Mark J Fogg, Vincent Fromion, Anne Goelzer, Annette Hansen, Elisabeth Härtig, Colin R Harwood, Georg Homuth, Hanne Jarmer, Matthieu Jules, Edda Klipp, Ludovic Le Chat, François Lecointe, Peter Lewis, Wolfram Liebermeister, Anika March, Ruben A T Mars, Priyanka Nannapaneni, David Noone, Susanne Pohl, Bernd Rinn, Frank Rügheimer, Praveen K Sappa, Franck Samson, Marc Schaffer, Benno Schwikowski, Leif Steil, Jörg Stülke, Thomas Wiegert, Kevin M Devine, Anthony J Wilkinson, Jan Maarten van Dijl, Michael Hecker, Uwe Völker, Philippe Bessières, Philippe Noirot
Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis.
Science: 2012, 335(6072);1103-6
[PubMed:22383849]
[WorldCat.org]
[DOI]
(I p)
Michael J Rudolph, Margot M Wuebbens, Oliver Turque, K V Rajagopalan, Hermann Schindelin
Structural studies of molybdopterin synthase provide insights into its catalytic mechanism.
J Biol Chem: 2003, 278(16);14514-22
[PubMed:12571227]
[WorldCat.org]
[DOI]
(P p)