Difference between revisions of "MntR"
| Line 127: | Line 127: | ||
** number of protein molecules per cell (minimal medium with glucose and ammonium): 376 {{PubMed|24696501}} | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 376 {{PubMed|24696501}} | ||
** number of protein molecules per cell (complex medium with amino acids, without glucose): 790 {{PubMed|24696501}} | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 790 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 900 {{PubMed|21395229}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 300 {{PubMed|21395229}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 488 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 14:10, 17 April 2014
- Description: transcriptional regulator, (repression of mntH and mntA-mntB-mntC-mntD under high Mn(II) conditions)
| Gene name | mntR |
| Synonyms | yqhN |
| Essential | no |
| Product | transcriptional regulator (DtxR family) |
| Function | regulation of manganese transport |
| Gene expression levels in SubtiExpress: mntR | |
| Metabolic function and regulation of this protein in SubtiPathways: mntR | |
| MW, pI | 16 kDa, 5.631 |
| Gene length, protein length | 426 bp, 142 aa |
| Immediate neighbours | yqhO, lipM |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 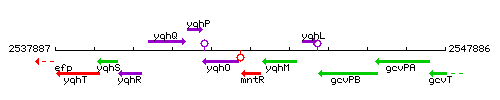
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed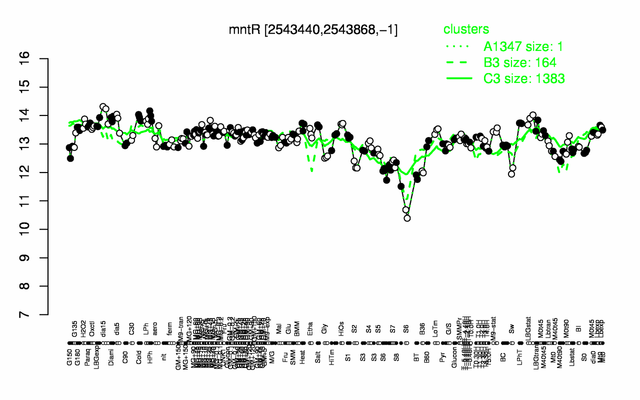
| |
Contents
Categories containing this gene/protein
trace metal homeostasis (Cu, Zn, Ni, Mn, Mo), transcription factors and their control, membrane proteins
This gene is a member of the following regulons
The MntR regulon:
The gene
Basic information
- Locus tag: BSU24520
Phenotypes of a mutant
Database entries
- BsubCyc: BSU24520
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Mn(2+) acts as co-repressor (according to PubMed)
- Effectors of protein activity:
- Interactions:
- active as dimer (according to PubMed)
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- BsubCyc: BSU24520
- UniProt: P54512
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 376 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 790 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 900 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 300 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 488 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Richard Brennan, Houston, Texas, USA Homepage
Your additional remarks
References
Reviews
Sabine Brantl, Andreas Licht
Characterisation of Bacillus subtilis transcriptional regulators involved in metabolic processes.
Curr Protein Pept Sci: 2010, 11(4);274-91
[PubMed:20408793]
[WorldCat.org]
[DOI]
(I p)
Original Publications
Amanda M McGuire, Bonnie J Cuthbert, Zhen Ma, Kristen D Grauer-Gray, Megan Brunjes Brophy, Kayce A Spear, Sumarin Soonsanga, Joseph I Kliegman, Sarah L Griner, John D Helmann, Arthur Glasfeld
Roles of the A and C sites in the manganese-specific activation of MntR.
Biochemistry: 2013, 52(4);701-13
[PubMed:23298157]
[WorldCat.org]
[DOI]
(I p)
Misha V Golynskiy, William A Gunderson, Michael P Hendrich, Seth M Cohen
Metal binding studies and EPR spectroscopy of the manganese transport regulator MntR.
Biochemistry: 2006, 45(51);15359-72
[PubMed:17176058]
[WorldCat.org]
[DOI]
(I p)
Mark A DeWitt, Joseph I Kliegman, John D Helmann, Richard G Brennan, David L Farrens, Arthur Glasfeld
The conformations of the manganese transport regulator of Bacillus subtilis in its metal-free state.
J Mol Biol: 2007, 365(5);1257-65
[PubMed:17118401]
[WorldCat.org]
[DOI]
(P p)
Joseph I Kliegman, Sarah L Griner, John D Helmann, Richard G Brennan, Arthur Glasfeld
Structural basis for the metal-selective activation of the manganese transport regulator of Bacillus subtilis.
Biochemistry: 2006, 45(11);3493-505
[PubMed:16533030]
[WorldCat.org]
[DOI]
(P p)
Misha V Golynskiy, Talib C Davis, John D Helmann, Seth M Cohen
Metal-induced structural organization and stabilization of the metalloregulatory protein MntR.
Biochemistry: 2005, 44(9);3380-9
[PubMed:15736948]
[WorldCat.org]
[DOI]
(P p)
Scot A Lieser, Talib C Davis, John D Helmann, Seth M Cohen
DNA-binding and oligomerization studies of the manganese(II) metalloregulatory protein MntR from Bacillus subtilis.
Biochemistry: 2003, 42(43);12634-42
[PubMed:14580210]
[WorldCat.org]
[DOI]
(P p)
Emmanuel Guedon, Charles M Moore, Qiang Que, Tao Wang, Rick W Ye, John D Helmann
The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons.
Mol Microbiol: 2003, 49(6);1477-91
[PubMed:12950915]
[WorldCat.org]
[DOI]
(P p)
Arthur Glasfeld, Emmanuel Guedon, John D Helmann, Richard G Brennan
Structure of the manganese-bound manganese transport regulator of Bacillus subtilis.
Nat Struct Biol: 2003, 10(8);652-7
[PubMed:12847518]
[WorldCat.org]
[DOI]
(P p)
Emmanuel Guedon, John D Helmann
Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators.
Mol Microbiol: 2003, 48(2);495-506
[PubMed:12675807]
[WorldCat.org]
[DOI]
(P p)
Q Que, J D Helmann
Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins.
Mol Microbiol: 2000, 35(6);1454-68
[PubMed:10760146]
[WorldCat.org]
[DOI]
(P p)