MleA
- Description: malic enzyme
| Gene name | mleA |
| Synonyms | yqkJ |
| Essential | no |
| Product | NAD-dependent malate dehydrogenase |
| Function | malate utilization |
| Gene expression levels in SubtiExpress: mleA | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 45 kDa, 4.895 |
| Gene length, protein length | 1317 bp, 439 aa |
| Immediate neighbours | yqkK, mleN |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 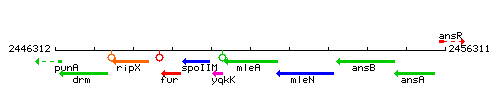
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed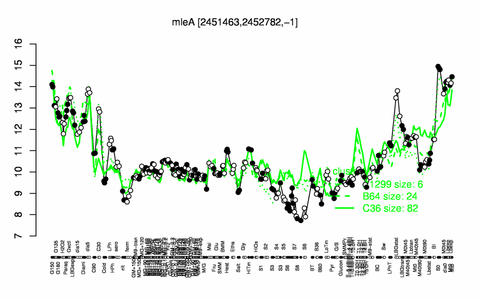
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU23550
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: (S)-malate + NAD+ = pyruvate + CO2 + NADH (according to Swiss-Prot) malate --> pyruvate
- Protein family: malic enzymes family (according to Swiss-Prot)
- Paralogous protein(s): YtsJ
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P54572
- KEGG entry: [2]
- E.C. number: 1.1.1.38
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant: GP1136 (cat) available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Frederik M Meyer, Jörg Stülke
Malate metabolism in Bacillus subtilis: distinct roles for three classes of malate-oxidizing enzymes.
FEMS Microbiol Lett: 2013, 339(1);17-22
[PubMed:23136871]
[WorldCat.org]
[DOI]
(I p)
Bogumiła C Marciniak, Monika Pabijaniak, Anne de Jong, Robert Dűhring, Gerald Seidel, Wolfgang Hillen, Oscar P Kuipers
High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis.
BMC Genomics: 2012, 13;401
[PubMed:22900538]
[WorldCat.org]
[DOI]
(I e)
Guillaume Lerondel, Thierry Doan, Nicola Zamboni, Uwe Sauer, Stéphane Aymerich
YtsJ has the major physiological role of the four paralogous malic enzyme isoforms in Bacillus subtilis.
J Bacteriol: 2006, 188(13);4727-36
[PubMed:16788182]
[WorldCat.org]
[DOI]
(P p)
Susan H Fisher, Lewis V Wray
Bacillus subtilis 168 contains two differentially regulated genes encoding L-asparaginase.
J Bacteriol: 2002, 184(8);2148-54
[PubMed:11914346]
[WorldCat.org]
[DOI]
(P p)