Mbl
- Description: cell shape-determining protein, forms filaments, the polymers control/restrict the mobility of the cell wall elongation enzyme complex, required for CwlO activity
| Gene name | mbl |
| Synonyms | |
| Essential | no |
| Product | MreB-like protein |
| Function | cell shape determination |
| Gene expression levels in SubtiExpress: mbl | |
| Interactions involving this protein in SubtInteract: Mbl | |
| MW, pI | 35 kDa, 5.669 |
| Gene length, protein length | 999 bp, 333 aa |
| Immediate neighbours | flhO, spoIIID |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed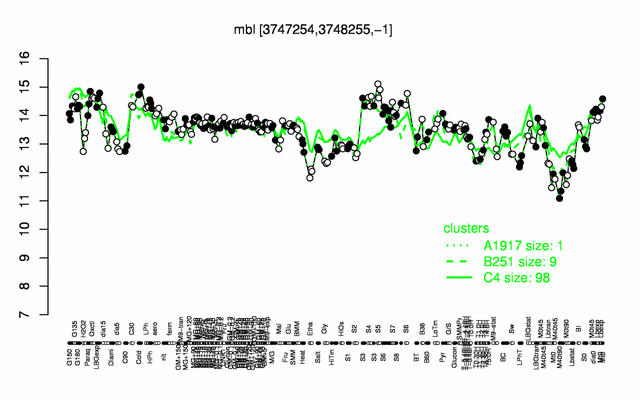
| |
Contents
Categories containing this gene/protein
cell shape, sporulation proteins, membrane proteins
This gene is a member of the following regulons
SigE regulon, stringent response
The gene
Basic information
- Locus tag: BSU36410
Phenotypes of a mutant
Database entries
- BsubCyc: BSU36410
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ftsA/mreB family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
- Localization:
- during logarithmic growth, Mbl forms discrete patches that move processively along peripheral tracks perpendicular to the cell axis PubMed
- forms transverse bands as cells enter the stationary phase PubMed
- close to the inner surface of the cytoplasmic membrane PubMed
- reports on helical structures formed by Mbl PubMed seem to be misinterpretation of data PubMed
Database entries
- BsubCyc: BSU36410
- Structure:
- UniProt: P39751
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- An antisense RNA is predicted for mbl PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 336 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 2526 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Görke lab
- Antibody: available in the Jeff Errington and Peter Graumann labs
Labs working on this gene/protein
Peter Graumann, Freiburg University, Germany homepage
Your additional remarks
References
Localization
Felix Dempwolff, Christian Reimold, Michael Reth, Peter L Graumann
Bacillus subtilis MreB orthologs self-organize into filamentous structures underneath the cell membrane in a heterologous cell system.
PLoS One: 2011, 6(11);e27035
[PubMed:22069484]
[WorldCat.org]
[DOI]
(I p)
Ethan C Garner, Remi Bernard, Wenqin Wang, Xiaowei Zhuang, David Z Rudner, Tim Mitchison
Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis.
Science: 2011, 333(6039);222-5
[PubMed:21636745]
[WorldCat.org]
[DOI]
(I p)
Julia Domínguez-Escobar, Arnaud Chastanet, Alvaro H Crevenna, Vincent Fromion, Roland Wedlich-Söldner, Rut Carballido-López
Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria.
Science: 2011, 333(6039);225-8
[PubMed:21636744]
[WorldCat.org]
[DOI]
(I p)
Henrik Strahl, Leendert W Hamoen
Membrane potential is important for bacterial cell division.
Proc Natl Acad Sci U S A: 2010, 107(27);12281-6
[PubMed:20566861]
[WorldCat.org]
[DOI]
(I p)
Hervé Joël Defeu Soufo, Peter L Graumann
Dynamic localization and interaction with other Bacillus subtilis actin-like proteins are important for the function of MreB.
Mol Microbiol: 2006, 62(5);1340-56
[PubMed:17064365]
[WorldCat.org]
[DOI]
(P p)
Other original publications
Katarína Muchová, Zuzana Chromiková, Imrich Barák
Control of Bacillus subtilis cell shape by RodZ.
Environ Microbiol: 2013, 15(12);3259-71
[PubMed:23879732]
[WorldCat.org]
[DOI]
(I p)
Patricia Domínguez-Cuevas, Ida Porcelli, Richard A Daniel, Jeff Errington
Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis.
Mol Microbiol: 2013, 89(6);1084-98
[PubMed:23869552]
[WorldCat.org]
[DOI]
(I p)
Siyuan Wang, Leon Furchtgott, Kerwyn Casey Huang, Joshua W Shaevitz
Helical insertion of peptidoglycan produces chiral ordering of the bacterial cell wall.
Proc Natl Acad Sci U S A: 2012, 109(10);E595-604
[PubMed:22343529]
[WorldCat.org]
[DOI]
(I p)
Erin Treece, Andrew Pinkham, Thomas Kim
Aminoguanidine down-regulates the expression of mreB-like protein in Bacillus subtilis.
Curr Microbiol: 2012, 64(2);112-7
[PubMed:22048160]
[WorldCat.org]
[DOI]
(I p)
Hervé Joël Defeu Soufo, Peter L Graumann
Bacillus subtilis MreB paralogues have different filament architectures and lead to shape remodelling of a heterologous cell system.
Mol Microbiol: 2010, 78(5);1145-58
[PubMed:21091501]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Yoshikazu Kawai, Kei Asai, Jeffery Errington
Partial functional redundancy of MreB isoforms, MreB, Mbl and MreBH, in cell morphogenesis of Bacillus subtilis.
Mol Microbiol: 2009, 73(4);719-31
[PubMed:19659933]
[WorldCat.org]
[DOI]
(I p)
Kathrin Schirner, Jeff Errington
Influence of heterologous MreB proteins on cell morphology of Bacillus subtilis.
Microbiology (Reading): 2009, 155(Pt 11);3611-3621
[PubMed:19643765]
[WorldCat.org]
[DOI]
(I p)
Kathrin Schirner, Jeff Errington
The cell wall regulator {sigma}I specifically suppresses the lethal phenotype of mbl mutants in Bacillus subtilis.
J Bacteriol: 2009, 191(5);1404-13
[PubMed:19114499]
[WorldCat.org]
[DOI]
(I p)
Rut Carballido-López, Alex Formstone, Ying Li, S Dusko Ehrlich, Philippe Noirot, Jeff Errington
Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE.
Dev Cell: 2006, 11(3);399-409
[PubMed:16950129]
[WorldCat.org]
[DOI]
(P p)
Kittichoat Tiyanont, Thierry Doan, Michael B Lazarus, Xiao Fang, David Z Rudner, Suzanne Walker
Imaging peptidoglycan biosynthesis in Bacillus subtilis with fluorescent antibiotics.
Proc Natl Acad Sci U S A: 2006, 103(29);11033-8
[PubMed:16832063]
[WorldCat.org]
[DOI]
(P p)
Mark Leaver, Jeff Errington
Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis.
Mol Microbiol: 2005, 57(5);1196-209
[PubMed:16101995]
[WorldCat.org]
[DOI]
(P p)
Richard A Daniel, Jeff Errington
Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell.
Cell: 2003, 113(6);767-76
[PubMed:12809607]
[WorldCat.org]
[DOI]
(P p)
Rut Carballido-López, Jeff Errington
The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis.
Dev Cell: 2003, 4(1);19-28
[PubMed:12530960]
[WorldCat.org]
[DOI]
(P p)
L J Jones, R Carballido-López, J Errington
Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis.
Cell: 2001, 104(6);913-22
[PubMed:11290328]
[WorldCat.org]
[DOI]
(P p)
A Decatur, M T McMurry, B N Kunkel, R Losick
Translation of the mRNA for the sporulation gene spoIIID of Bacillus subtilis is dependent upon translation of a small upstream open reading frame.
J Bacteriol: 1997, 179(4);1324-8
[PubMed:9023218]
[WorldCat.org]
[DOI]
(P p)
Y Abhayawardhane, G C Stewart
Bacillus subtilis possesses a second determinant with extensive sequence similarity to the Escherichia coli mreB morphogene.
J Bacteriol: 1995, 177(3);765-73
[PubMed:7836311]
[WorldCat.org]
[DOI]
(P p)