Difference between revisions of "LonA"
| Line 37: | Line 37: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| Line 115: | Line 111: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=lonA_2880466_2882790_-1 lonA] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=lonA_2880466_2882790_-1 lonA] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' [[SigA]] {{PubMed|7961402}} | + | * '''[[Sigma factor]]:''' [[SigA]] {{PubMed|7961402}} |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 144: | Line 140: | ||
=References= | =References= | ||
| − | + | ==Reviews== | |
| + | '''Additional reviews:''' {{PubMed|23479438}} | ||
| + | ==Original publications== | ||
<pubmed>7961403,7961402, 19542270, 19643080 9852015 18689473 20600124 </pubmed> | <pubmed>7961403,7961402, 19542270, 19643080 9852015 18689473 20600124 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:36, 14 March 2013
- Description: class III heat-shock ATP-dependent protease
| Gene name | lonA |
| Synonyms | lon |
| Essential | no |
| Product | class III heat-shock ATP-dependent protease |
| Function | protein quality control |
| Gene expression levels in SubtiExpress: lonA | |
| Regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 86 kDa, 5.774 |
| Gene length, protein length | 2322 bp, 774 aa |
| Immediate neighbours | ysxC, lonB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 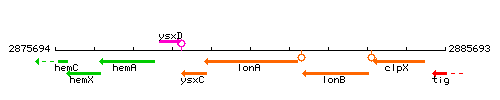
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed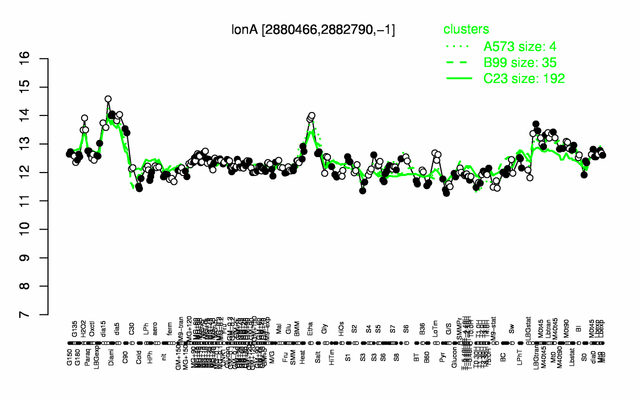
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU28200
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
A mutation in lonA suppresses the motility defect of a lytC mutant PubMed
Evidence for a specific DNA binding activitity of the α-domain was found PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Hydrolysis of proteins in presence of ATP (according to Swiss-Prot)
- Protein family: peptidase S16 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- homohexameric protein PubMed
- Localization: coincident with the nucleoid during normal growth and localized to the forespore during development PubMed
Database entries
- UniProt: P37945
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Additional reviews: PubMed
Original publications
Ramona E Duman, Jan Löwe
Crystal structures of Bacillus subtilis Lon protease.
J Mol Biol: 2010, 401(4);653-70
[PubMed:20600124]
[WorldCat.org]
[DOI]
(I p)
Yu-Ching Lin, Huai-Cheng Lee, Iren Wang, Chun-Hua Hsu, Jiahn-Haur Liao, Alan Yueh-Luen Lee, Chinpan Chen, Shih-Hsiung Wu
DNA-binding specificity of the Lon protease alpha-domain from Brevibacillus thermoruber WR-249.
Biochem Biophys Res Commun: 2009, 388(1);62-6
[PubMed:19643080]
[WorldCat.org]
[DOI]
(I p)
Rui Chen, Sarah B Guttenplan, Kris M Blair, Daniel B Kearns
Role of the sigmaD-dependent autolysins in Bacillus subtilis population heterogeneity.
J Bacteriol: 2009, 191(18);5775-84
[PubMed:19542270]
[WorldCat.org]
[DOI]
(I p)
Lyle A Simmons, Alan D Grossman, Graham C Walker
Clp and Lon proteases occupy distinct subcellular positions in Bacillus subtilis.
J Bacteriol: 2008, 190(20);6758-68
[PubMed:18689473]
[WorldCat.org]
[DOI]
(I p)
E Krüger, M Hecker
The first gene of the Bacillus subtilis clpC operon, ctsR, encodes a negative regulator of its own operon and other class III heat shock genes.
J Bacteriol: 1998, 180(24);6681-8
[PubMed:9852015]
[WorldCat.org]
[DOI]
(P p)
R Schmidt, A L Decatur, P N Rather, C P Moran, R Losick
Bacillus subtilis lon protease prevents inappropriate transcription of genes under the control of the sporulation transcription factor sigma G.
J Bacteriol: 1994, 176(21);6528-37
[PubMed:7961403]
[WorldCat.org]
[DOI]
(P p)
S Riethdorf, U Völker, U Gerth, A Winkler, S Engelmann, M Hecker
Cloning, nucleotide sequence, and expression of the Bacillus subtilis lon gene.
J Bacteriol: 1994, 176(21);6518-27
[PubMed:7961402]
[WorldCat.org]
[DOI]
(P p)