LiaR
- Description: two-component response regulator, regulation of the liaI-liaH-liaG-liaF-liaS-liaR operon in response to bacitracin
| Gene name | liaR |
| Synonyms | yvqC |
| Essential | no |
| Product | two-component response regulator |
| Function | regulation of the liaI-liaH-liaG-liaF-liaS-liaR operon in response to bacitracin |
| Gene expression levels in SubtiExpress: liaR
operon in response to bacitracin | |
| Interactions involving this protein in SubtInteract: LiaR | |
| MW, pI | 22 kDa, 4.956 |
| Gene length, protein length | 633 bp, 211 aa |
| Immediate neighbours | gerAC, liaS |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed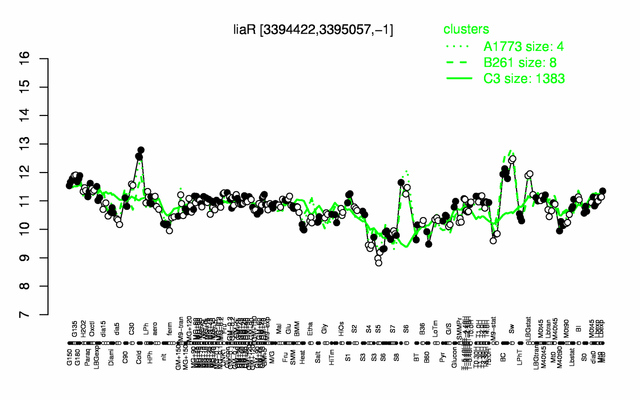
| |
Contents
Categories containing this gene/protein
transcription factors and their control, resistance against oxidative and electrophile stress, resistance against toxins/ antibiotics, phosphoproteins
This gene is a member of the following regulons
The LiaR regulon
The gene
Basic information
- Locus tag: BSU33080
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: regulation of the liaI-liaH-liaG-liaF-liaS-liaR operon in response to bacitracin
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on a Asp residue by LiaS
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O32197
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Additional publications: PubMed
Karen Schrecke, Sina Jordan, Thorsten Mascher
Stoichiometry and perturbation studies of the LiaFSR system of Bacillus subtilis.
Mol Microbiol: 2013, 87(4);769-88
[PubMed:23279150]
[WorldCat.org]
[DOI]
(I p)
Diana Wolf, Falk Kalamorz, Tina Wecke, Anna Juszczak, Ulrike Mäder, Georg Homuth, Sina Jordan, Janine Kirstein, Michael Hoppert, Birgit Voigt, Michael Hecker, Thorsten Mascher
In-depth profiling of the LiaR response of Bacillus subtilis.
J Bacteriol: 2010, 192(18);4680-93
[PubMed:20639339]
[WorldCat.org]
[DOI]
(I p)
Andriansjah Rukmana, Takuya Morimoto, Hiroki Takahashi, Giyanto, Naotake Ogasawara
Assessment of transcriptional responses of Bacillus subtilis cells to the antibiotic enduracidin, which interferes with cell wall synthesis, using a high-density tiling chip.
Genes Genet Syst: 2009, 84(4);253-67
[PubMed:20057163]
[WorldCat.org]
[DOI]
(P p)
Tina Wecke, Daniela Zühlke, Ulrike Mäder, Sina Jordan, Birgit Voigt, Stefan Pelzer, Harald Labischinski, Georg Homuth, Michael Hecker, Thorsten Mascher
Daptomycin versus Friulimicin B: in-depth profiling of Bacillus subtilis cell envelope stress responses.
Antimicrob Agents Chemother: 2009, 53(4);1619-23
[PubMed:19164157]
[WorldCat.org]
[DOI]
(I p)
Anna-Barbara Hachmann, Esther R Angert, John D Helmann
Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin.
Antimicrob Agents Chemother: 2009, 53(4);1598-609
[PubMed:19164152]
[WorldCat.org]
[DOI]
(I p)
Eva Rietkötter, Diana Hoyer, Thorsten Mascher
Bacitracin sensing in Bacillus subtilis.
Mol Microbiol: 2008, 68(3);768-85
[PubMed:18394148]
[WorldCat.org]
[DOI]
(I p)
Bronwyn G Butcher, Yi-Pin Lin, John D Helmann
The yydFGHIJ operon of Bacillus subtilis encodes a peptide that induces the LiaRS two-component system.
J Bacteriol: 2007, 189(23);8616-25
[PubMed:17921301]
[WorldCat.org]
[DOI]
(I p)
Sina Jordan, Eva Rietkötter, Mark A Strauch, Falk Kalamorz, Bronwyn G Butcher, John D Helmann, Thorsten Mascher
LiaRS-dependent gene expression is embedded in transition state regulation in Bacillus subtilis.
Microbiology (Reading): 2007, 153(Pt 8);2530-2540
[PubMed:17660417]
[WorldCat.org]
[DOI]
(P p)
Hanne-Leena Hyyryläinen, Milla Pietiäinen, Tuula Lundén, Anna Ekman, Marika Gardemeister, Sanna Murtomäki-Repo, Haike Antelmann, Michael Hecker, Leena Valmu, Matti Sarvas, Vesa P Kontinen
The density of negative charge in the cell wall influences two-component signal transduction in Bacillus subtilis.
Microbiology (Reading): 2007, 153(Pt 7);2126-2136
[PubMed:17600057]
[WorldCat.org]
[DOI]
(P p)
Sina Jordan, Anja Junker, John D Helmann, Thorsten Mascher
Regulation of LiaRS-dependent gene expression in bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system.
J Bacteriol: 2006, 188(14);5153-66
[PubMed:16816187]
[WorldCat.org]
[DOI]
(P p)
Thorsten Mascher, Sara L Zimmer, Terry-Ann Smith, John D Helmann
Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis.
Antimicrob Agents Chemother: 2004, 48(8);2888-96
[PubMed:15273097]
[WorldCat.org]
[DOI]
(P p)
Mélanie A Hamon, Nicola R Stanley, Robert A Britton, Alan D Grossman, Beth A Lazazzera
Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis.
Mol Microbiol: 2004, 52(3);847-60
[PubMed:15101989]
[WorldCat.org]
[DOI]
(P p)
Thorsten Mascher, Neil G Margulis, Tao Wang, Rick W Ye, John D Helmann
Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon.
Mol Microbiol: 2003, 50(5);1591-604
[PubMed:14651641]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)