Difference between revisions of "KinC"
| Line 1: | Line 1: | ||
| − | * '''Description:''' [[two-component systems|two-component]] sensor kinase, phosphorylates [[Spo0F]] and [[Spo0A]], part of the [[phosphorelay]], governs expression of genes involved in [[biofilm formation]] <br/><br/> | + | * '''Description:''' [[two-component systems|two-component]] sensor kinase, phosphorylates [[Spo0F]] and [[Spo0A]] in response to the presence of surfactin, part of the [[phosphorelay]], governs expression of genes involved in [[biofilm formation]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 12: | Line 12: | ||
|style="background:#ABCDEF;" align="center"| '''Product''' || [[two-component systems|two-component]] sensor kinase | |style="background:#ABCDEF;" align="center"| '''Product''' || [[two-component systems|two-component]] sensor kinase | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Function''' || initiation of sporulation | + | |style="background:#ABCDEF;" align="center"|'''Function''' || initiation of [[sporulation]] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU14490 kinC] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU14490 kinC] | ||
| Line 36: | Line 36: | ||
__TOC__ | __TOC__ | ||
| − | |||
| − | |||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
<br/><br/><br/><br/><br/><br/> | <br/><br/><br/><br/><br/><br/> | ||
| Line 49: | Line 46: | ||
{{SubtiWiki category|[[transcription factors and their control]]}}, | {{SubtiWiki category|[[transcription factors and their control]]}}, | ||
{{SubtiWiki category|[[phosphorelay]]}}, | {{SubtiWiki category|[[phosphorelay]]}}, | ||
| + | {{SubtiWiki category|[[biofilm formation]]}}, | ||
{{SubtiWiki category|[[membrane proteins]]}}, | {{SubtiWiki category|[[membrane proteins]]}}, | ||
{{SubtiWiki category|[[phosphoproteins]]}} | {{SubtiWiki category|[[phosphoproteins]]}} | ||
| Line 62: | Line 60: | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| + | ** defective in [[biofilm formation]] {{PubMed|22882210}} | ||
=== Database entries === | === Database entries === | ||
| Line 70: | Line 69: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| Line 81: | Line 78: | ||
** autophosphorylation, phosphorylation of [[Spo0F]] as part of the [[phosphorelay]], but also direct phosphorylation of [[Spo0A]] {{PubMed|19114652}} | ** autophosphorylation, phosphorylation of [[Spo0F]] as part of the [[phosphorelay]], but also direct phosphorylation of [[Spo0A]] {{PubMed|19114652}} | ||
** mainly active in the younger, outer regions of a colony (with [[KinD]]) {{PubMed|21097618}} | ** mainly active in the younger, outer regions of a colony (with [[KinD]]) {{PubMed|21097618}} | ||
| + | ** phosphorylates [[Spo0A]] in response to the presence of surfactin {{PubMed|22882210}} | ||
* '''Protein family:''' | * '''Protein family:''' | ||
| Line 107: | Line 105: | ||
** cell membrane (Heterogeneous) [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] | ** cell membrane (Heterogeneous) [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] | ||
** co-localizes with [[FloT]] in discrete foci in the membrane {{PubMed|20713508}} | ** co-localizes with [[FloT]] in discrete foci in the membrane {{PubMed|20713508}} | ||
| + | ** the localization of [[KinC]] in membrane microdomains depends on [[FloA]] and [[FloT]] {{PubMed|22882210}} | ||
=== Database entries === | === Database entries === | ||
| Line 156: | Line 155: | ||
=References= | =References= | ||
| − | <pubmed>19114652,10094672,11069677,16166384, 20713508,8002615, 16479537 8002614 20946851 20971918 21097618 </pubmed> | + | <pubmed>19114652,10094672,11069677,16166384, 20713508,8002615, 16479537 8002614 20946851 20971918 21097618 22882210</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 12:58, 14 August 2012
- Description: two-component sensor kinase, phosphorylates Spo0F and Spo0A in response to the presence of surfactin, part of the phosphorelay, governs expression of genes involved in biofilm formation
| Gene name | kinC |
| Synonyms | ssb |
| Essential | no |
| Product | two-component sensor kinase |
| Function | initiation of sporulation |
| Gene expression levels in SubtiExpress: kinC | |
| Interactions involving this protein in SubtInteract: KinC | |
| Function and regulation of this protein in SubtiPathways: Phosphorelay | |
| MW, pI | 48 kDa, 6.225 |
| Gene length, protein length | 1284 bp, 428 aa |
| Immediate neighbours | abh, ykqA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 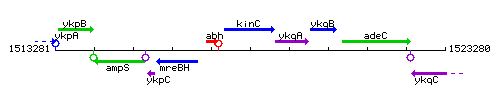
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed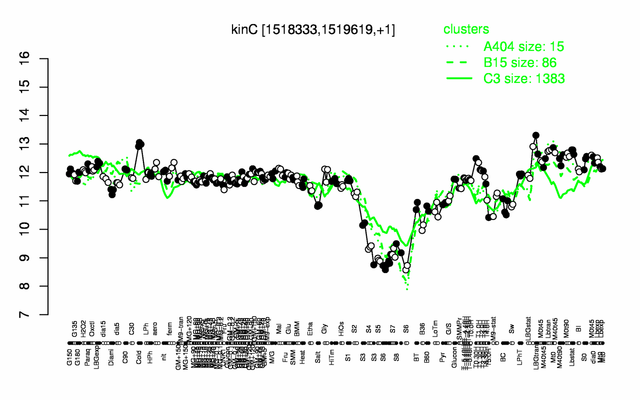
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control, phosphorelay, biofilm formation, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14490
Phenotypes of a mutant
- defective in biofilm formation PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains: two transmembrane segments, C-terminal histidine phosphotransferase domain
- Modification: autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P39764
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: kinC (according to DBTBS)
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ana Yepes, Johannes Schneider, Benjamin Mielich, Gudrun Koch, Juan-Carlos García-Betancur, Kumaran S Ramamurthi, Hera Vlamakis, Daniel López
The biofilm formation defect of a Bacillus subtilis flotillin-defective mutant involves the protease FtsH.
Mol Microbiol: 2012, 86(2);457-71
[PubMed:22882210]
[WorldCat.org]
[DOI]
(I p)
Anna L McLoon, Ilana Kolodkin-Gal, Shmuel M Rubinstein, Roberto Kolter, Richard Losick
Spatial regulation of histidine kinases governing biofilm formation in Bacillus subtilis.
J Bacteriol: 2011, 193(3);679-85
[PubMed:21097618]
[WorldCat.org]
[DOI]
(I p)
Moshe Shemesh, Roberto Kolter, Richard Losick
The biocide chlorine dioxide stimulates biofilm formation in Bacillus subtilis by activation of the histidine kinase KinC.
J Bacteriol: 2010, 192(24);6352-6
[PubMed:20971918]
[WorldCat.org]
[DOI]
(I p)
Daniel López, Erin A Gontang, Roberto Kolter
Potassium sensing histidine kinase in Bacillus subtilis.
Methods Enzymol: 2010, 471;229-51
[PubMed:20946851]
[WorldCat.org]
[DOI]
(I p)
Daniel López, Roberto Kolter
Functional microdomains in bacterial membranes.
Genes Dev: 2010, 24(17);1893-902
[PubMed:20713508]
[WorldCat.org]
[DOI]
(I p)
Daniel López, Michael A Fischbach, Frances Chu, Richard Losick, Roberto Kolter
Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis.
Proc Natl Acad Sci U S A: 2009, 106(1);280-5
[PubMed:19114652]
[WorldCat.org]
[DOI]
(I p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Masaya Fujita, Richard Losick
Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A.
Genes Dev: 2005, 19(18);2236-44
[PubMed:16166384]
[WorldCat.org]
[DOI]
(P p)
M Jiang, W Shao, M Perego, J A Hoch
Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis.
Mol Microbiol: 2000, 38(3);535-42
[PubMed:11069677]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)
K Kobayashi, K Shoji, T Shimizu, K Nakano, T Sato, Y Kobayashi
Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase.
J Bacteriol: 1995, 177(1);176-82
[PubMed:8002615]
[WorldCat.org]
[DOI]
(P p)
J R LeDeaux, A D Grossman
Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis.
J Bacteriol: 1995, 177(1);166-75
[PubMed:8002614]
[WorldCat.org]
[DOI]
(P p)