Difference between revisions of "IolA"
(→References) |
|||
| Line 147: | Line 147: | ||
=References= | =References= | ||
'''Additional publications:''' {{PubMed|22782904,21515690}} | '''Additional publications:''' {{PubMed|22782904,21515690}} | ||
| − | <pubmed>9226270,11566986,10666464,18310071,9887260,,9887260,18310071, 16332250</pubmed> | + | <pubmed>9226270,11566986,10666464,18310071,9887260,,9887260,18310071, 16332250 22900538 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:06, 23 August 2012
- Description: methylmalonate-semialdehyde dehydrogenase (acylating)
| Gene name | iolA |
| Synonyms | mmsA, yxdA |
| Essential | no |
| Product | methylmalonate-semialdehyde dehydrogenase (acylating) |
| Function | myo-inositol catabolism |
| Gene expression levels in SubtiExpress: iolA | |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism | |
| MW, pI | 53 kDa, 5.139 |
| Gene length, protein length | 1461 bp, 487 aa |
| Immediate neighbours | iolB, iolR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 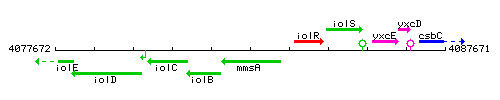
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed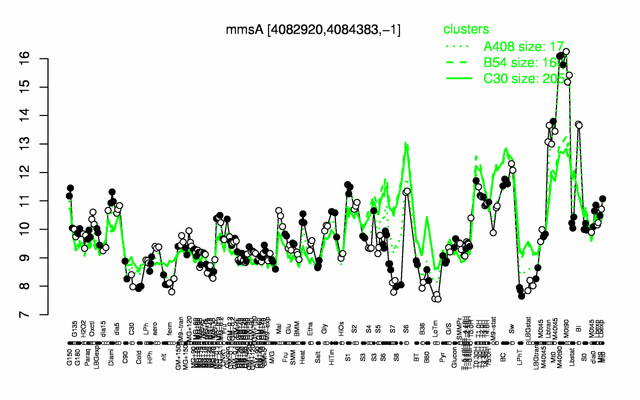
| |
Contents
Categories containing this gene/protein
utilization of specific carbon sources
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU39760
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: NAD-dependent oxidation of methylmalonate semialdehyde (MMSA) to propionyl-CoA via acylation and deacylation steps
- Protein family: IolA subfamily (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Coenzyme A, NAD PubMed
- Effectors of protein activity:
Database entries
- UniProt: P42412
- KEGG entry: [3]
- E.C. number: 1.2.1.27
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Yasutaro Fujita, University of Fukuyama, Japan
- Ken-ichi Yoshida, Kobe University, Japan
Your additional remarks
References
Additional publications: PubMed
Bogumiła C Marciniak, Monika Pabijaniak, Anne de Jong, Robert Dűhring, Gerald Seidel, Wolfgang Hillen, Oscar P Kuipers
High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis.
BMC Genomics: 2012, 13;401
[PubMed:22900538]
[WorldCat.org]
[DOI]
(I e)
Ken-ichi Yoshida, Masanori Yamaguchi, Tetsuro Morinaga, Masaki Kinehara, Maya Ikeuchi, Hitoshi Ashida, Yasutaro Fujita
myo-Inositol catabolism in Bacillus subtilis.
J Biol Chem: 2008, 283(16);10415-24
[PubMed:18310071]
[WorldCat.org]
[DOI]
(P p)
Claire Stines-Chaumeil, François Talfournier, Guy Branlant
Mechanistic characterization of the MSDH (methylmalonate semialdehyde dehydrogenase) from Bacillus subtilis.
Biochem J: 2006, 395(1);107-15
[PubMed:16332250]
[WorldCat.org]
[DOI]
(I p)
Y Miwa, Y Fujita
Involvement of two distinct catabolite-responsive elements in catabolite repression of the Bacillus subtilis myo-inositol (iol) operon.
J Bacteriol: 2001, 183(20);5877-84
[PubMed:11566986]
[WorldCat.org]
[DOI]
(P p)
Y Miwa, A Nakata, A Ogiwara, M Yamamoto, Y Fujita
Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis.
Nucleic Acids Res: 2000, 28(5);1206-10
[PubMed:10666464]
[WorldCat.org]
[DOI]
(I p)
K I Yoshida, T Shibayama, D Aoyama, Y Fujita
Interaction of a repressor and its binding sites for regulation of the Bacillus subtilis iol divergon.
J Mol Biol: 1999, 285(3);917-29
[PubMed:9887260]
[WorldCat.org]
[DOI]
(P p)
K I Yoshida, D Aoyama, I Ishio, T Shibayama, Y Fujita
Organization and transcription of the myo-inositol operon, iol, of Bacillus subtilis.
J Bacteriol: 1997, 179(14);4591-8
[PubMed:9226270]
[WorldCat.org]
[DOI]
(P p)