Difference between revisions of "HemH"
| Line 1: | Line 1: | ||
| − | * '''Description:''' ferrochelatase <br/><br/> | + | * '''Description:''' ferrochelatase, catalyzes the final step of heme b biosynthesis <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
|- | |- | ||
| Line 36: | Line 36: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | |||
| − | |||
| − | |||
| − | |||
<br/><br/> | <br/><br/> | ||
| Line 64: | Line 60: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 114: | Line 107: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=hemH_1087250_1088182_1 hemH] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=hemH_1087250_1088182_1 hemH] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 146: | Line 139: | ||
=References= | =References= | ||
| − | <pubmed>9384565,1459957,8119288, 21052751 23097001 16453119 </pubmed> | + | <pubmed>9384565,1459957,8119288, 21052751 23097001 16453119 25826316</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:27, 1 April 2015
- Description: ferrochelatase, catalyzes the final step of heme b biosynthesis
| Gene name | hemH |
| Synonyms | hemF |
| Essential | no |
| Product | ferrochelatase |
| Function | heme biosynthesis |
| Gene expression levels in SubtiExpress: hemH | |
| Metabolic function and regulation of this protein in SubtiPathways: HemH | |
| MW, pI | 35 kDa, 4.617 |
| Gene length, protein length | 930 bp, 310 aa |
| Immediate neighbours | hemE, hemY |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 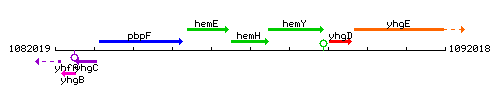
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed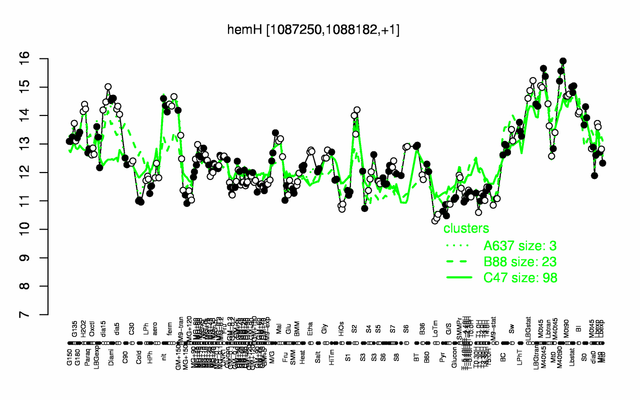
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU10130
Phenotypes of a mutant
Database entries
- BsubCyc: BSU10130
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: insertion of Fe(2+) into protoporphyrin IX
- Protein family: ferrochelatase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU10130
- UniProt: P32396
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 83 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 1426 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1547 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 1289 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1239 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Andreas Mielcarek, Bastian Blauenburg, Marcus Miethke, Mohamed A Marahiel
Molecular insights into frataxin-mediated iron supply for heme biosynthesis in Bacillus subtilis.
PLoS One: 2015, 10(3);e0122538
[PubMed:25826316]
[WorldCat.org]
[DOI]
(I e)
Yaxue Wang, Yong Shen
Is it possible for Fe2+ to approach protoporphyrin IX from the side of Tyr-13 in Bacillus subtilis ferrochelatase? An answer from QM/MM study.
J Mol Model: 2013, 19(2);963-71
[PubMed:23097001]
[WorldCat.org]
[DOI]
(I p)
Mattias D Hansson, Tobias Karlberg, Christopher A G Söderberg, Sreekanth Rajan, Martin J Warren, Salam Al-Karadaghi, Stephen E J Rigby, Mats Hansson
Bacterial ferrochelatase turns human: Tyr13 determines the apparent metal specificity of Bacillus subtilis ferrochelatase.
J Biol Inorg Chem: 2011, 16(2);235-42
[PubMed:21052751]
[WorldCat.org]
[DOI]
(I p)
Mattias D Hansson, Mats Lindstam, Mats Hansson
Crosstalk between metal ions in Bacillus subtilis ferrochelatase.
J Biol Inorg Chem: 2006, 11(3);325-33
[PubMed:16453119]
[WorldCat.org]
[DOI]
(P p)
S Al-Karadaghi, M Hansson, S Nikonov, B Jönsson, L Hederstedt
Crystal structure of ferrochelatase: the terminal enzyme in heme biosynthesis.
Structure: 1997, 5(11);1501-10
[PubMed:9384565]
[WorldCat.org]
[DOI]
(P p)
M Hansson, L Hederstedt
Purification and characterisation of a water-soluble ferrochelatase from Bacillus subtilis.
Eur J Biochem: 1994, 220(1);201-8
[PubMed:8119288]
[WorldCat.org]
[DOI]
(P p)
M Hansson, L Hederstedt
Cloning and characterization of the Bacillus subtilis hemEHY gene cluster, which encodes protoheme IX biosynthetic enzymes.
J Bacteriol: 1992, 174(24);8081-93
[PubMed:1459957]
[WorldCat.org]
[DOI]
(P p)