Difference between revisions of "GroEL"
Raphael2215 (talk | contribs) |
|||
| Line 23: | Line 23: | ||
|style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1632 bp, 544 aa | |style="background:#ABCDEF;" align="center"| '''Gene length, protein length''' || 1632 bp, 544 aa | ||
|- | |- | ||
| − | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[groES]]'', ''[[ | + | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[groES]]'', ''[[ydzT/1]]'' |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB12422]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | |colspan="2" style="background:#FAF8CC;" align="center"|'''Get the DNA and protein [http://srs.ebi.ac.uk/srsbin/cgi-bin/wgetz?-e+[EMBLCDS:CAB12422]+-newId sequences] <br/> (Barbe ''et al.'', 2009)''' | ||
Revision as of 11:15, 29 November 2012
- Description: chaperonin and co-repressor for HrcA
| Gene name | groEL |
| Synonyms | |
| Essential | yes PubMed |
| Product | chaperonin, co-repressor for HrcA |
| Function | protein folding and re-folding |
| Gene expression levels in SubtiExpress: groEL | |
| Interactions involving this protein in SubtInteract: GroEL | |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 57 kDa, 4.531 |
| Gene length, protein length | 1632 bp, 544 aa |
| Immediate neighbours | groES, ydzT/1 |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 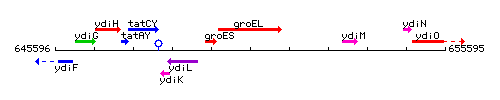
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed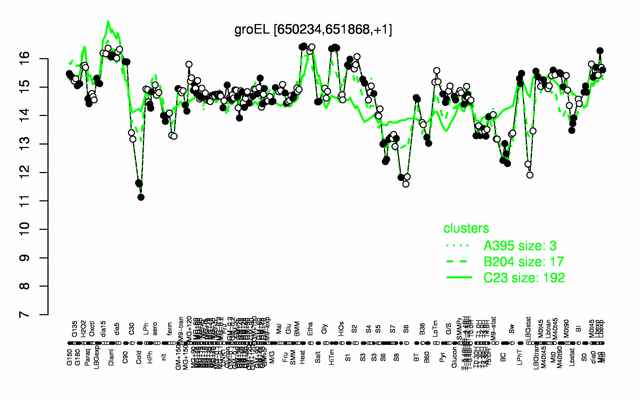
| |
Contents
Categories containing this gene/protein
chaperones/ protein folding, heat shock proteins, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU06030
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: acts as co-repressor for HrcA
- Protein family: chaperonin (HSP60) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cytoplasm (according to Swiss-Prot)
- recruited to the membrane after ethanol stress PubMed
Database entries
- Structure:
- UniProt: P28598
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Wolfgang Schumann, Bayreuth University, Germany Homepage
Your additional remarks
References
Additional publications: PubMed
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Christine Eymann, Dörte Becher, Jörg Bernhardt, Katrin Gronau, Anja Klutzny, Michael Hecker
Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis.
Proteomics: 2007, 7(19);3509-26
[PubMed:17726680]
[WorldCat.org]
[DOI]
(P p)
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Silke Reischl, Thomas Wiegert, Wolfgang Schumann
Isolation and analysis of mutant alleles of the Bacillus subtilis HrcA repressor with reduced dependency on GroE function.
J Biol Chem: 2002, 277(36);32659-67
[PubMed:12082092]
[WorldCat.org]
[DOI]
(P p)
A Mogk, G Homuth, C Scholz, L Kim, F X Schmid, W Schumann
The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis.
EMBO J: 1997, 16(15);4579-90
[PubMed:9303302]
[WorldCat.org]
[DOI]
(P p)
A Schulz, W Schumann
hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes.
J Bacteriol: 1996, 178(4);1088-93
[PubMed:8576042]
[WorldCat.org]
[DOI]
(P p)
G Yuan, S L Wong
Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK.
J Bacteriol: 1995, 177(22);6462-8
[PubMed:7592421]
[WorldCat.org]
[DOI]
(P p)
A Schmidt, M Schiesswohl, U Völker, M Hecker, W Schumann
Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis.
J Bacteriol: 1992, 174(12);3993-9
[PubMed:1350777]
[WorldCat.org]
[DOI]
(P p)
M Li, S L Wong
Cloning and characterization of the groESL operon from Bacillus subtilis.
J Bacteriol: 1992, 174(12);3981-92
[PubMed:1350776]
[WorldCat.org]
[DOI]
(P p)