Difference between revisions of "GroEL"
(→Basic information/ Evolution) |
|||
| Line 54: | Line 54: | ||
=== Basic information/ Evolution === | === Basic information/ Evolution === | ||
| − | * '''Catalyzed reaction/ biological activity:''' | + | * '''Catalyzed reaction/ biological activity:''' acts as co-repressor for [[HrcA]] |
* '''Protein family:''' chaperonin (HSP60) family (according to Swiss-Prot) | * '''Protein family:''' chaperonin (HSP60) family (according to Swiss-Prot) | ||
Revision as of 07:18, 31 May 2009
- Description: chaperonin
| Gene name | groEL |
| Synonyms | |
| Essential | yes PubMed |
| Product | chaperonin |
| Function | protein folding and re-folding |
| MW, pI | 57 kDa, 4.531 |
| Gene length, protein length | 1632 bp, 544 aa |
| Immediate neighbours | groES, ydiM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 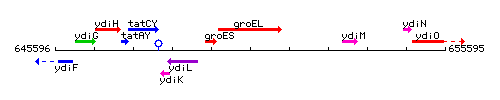
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: acts as co-repressor for HrcA
- Protein family: chaperonin (HSP60) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- Swiss prot entry: P28598
- KEGG entry: BSU06030
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Wolfgang Schumann, Bayreuth University, Germany Homepage
Your additional remarks
References
- Eymann et al. (2007) Dynamics of protein phosphorylation on Ser/Thr/Tyr in Bacillus subtilis. Proteomics 7: 3509-3526. PubMed
- Lévine et al. (2006) Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes. Proteomics 6: 2157-2173 PubMed
- Mogk, A., Homuth, G., Scholz, C., Kim, L., Schmid, F. X., and Schumann, W. (1997) The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J 16, 4579-4590. PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed