Difference between revisions of "GndA"
m (Reverted edits by 134.76.70.252 (talk) to last revision by Jstuelk) |
|||
| Line 100: | Line 100: | ||
* '''Operon:''' ''[[polY1]]-[[gndA]]'' (according to [http://dbtbs.hgc.jp/COG/prom/yqjHI.html DBTBS]) | * '''Operon:''' ''[[polY1]]-[[gndA]]'' (according to [http://dbtbs.hgc.jp/COG/prom/yqjHI.html DBTBS]) | ||
| − | * '''[ | + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=gndA_2480750_2482159_-1 gndA] {{PubMed|22383849}} |
| + | |||
| + | * '''Sigma factor:''' | ||
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 106: | Line 108: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
Revision as of 10:44, 16 April 2012
- Description: NADP-dependent phosphogluconate dehydrogenase
| Gene name | gndA |
| Synonyms | yqjI |
| Essential | no |
| Product | NADP-dependent phosphogluconate dehydrogenase |
| Function | pentose phosphate pathway |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 51 kDa, 5.076 |
| Gene length, protein length | 1407 bp, 469 aa |
| Immediate neighbours | zwf, polY1 |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 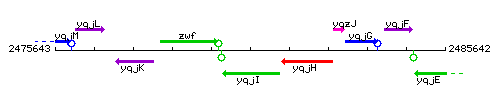
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
carbon core metabolism, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU23860
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 6-phospho-D-gluconate + NADP+ = D-ribulose 5-phosphate + CO2 + NADPH (according to Swiss-Prot)
- Protein family: 6-phosphogluconate dehydrogenase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: Reversible reaction PubMed
- Domains:
- Modification: phosphorylation on (Thr-15 OR Thr-17) PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P80859
- KEGG entry: [3]
- E.C. number: 1.1.1.44
Additional information
It contains a cysteine on the active site. The enzyme is a dimer. PubMed
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP1514 (gndA::kan), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
- FLAG-tag construct:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Scott Cameron, Viviane P Martini, Jorge Iulek, William N Hunter
Geobacillus stearothermophilus 6-phosphogluconate dehydrogenase complexed with 6-phosphogluconate.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2009, 65(Pt 5);450-4
[PubMed:19407374]
[WorldCat.org]
[DOI]
(I p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)