Gmk
- Description: guanylate kinase (GMP:dATP, dGMP:ATP)
| Gene name | gmk |
| Synonyms | yloD |
| Essential | yes PubMed |
| Product | guanylate kinase (GMP:dATP, dGMP:ATP) |
| Function | GTP biosynthesis |
| Metabolic function and regulation of this protein in SubtiPathways: Purine salvage, Nucleotides (regulation) | |
| MW, pI | 23 kDa, 4.616 |
| Gene length, protein length | 612 bp, 204 aa |
| Immediate neighbours | ylzA, yloH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 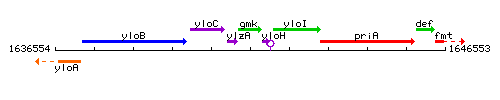
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed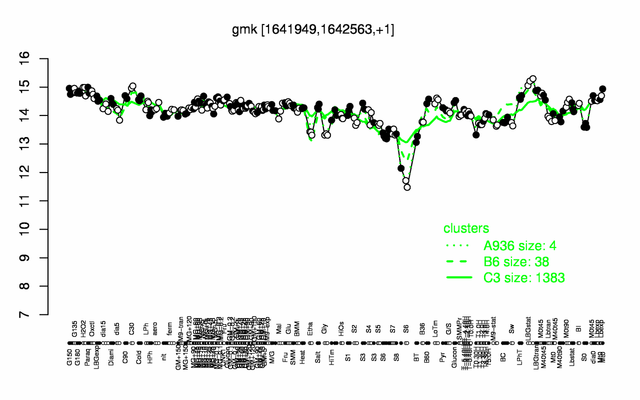
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of nucleotides, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15680
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + GMP = ADP + GDP (according to Swiss-Prot)
- Protein family: guanylate kinase-like domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-134 and/or Arg-149PubMed
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 1S4Q (from Mycobacterium tuberculosis h37rv, 39% identity, 60% similarity)
- UniProt: O34328
- KEGG entry: [2]
- E.C. number: 2.7.4.8
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)