GltA
Revision as of 15:07, 17 April 2014 by 134.76.70.252 (talk)
- Description: large subunit of glutamate synthase
| Gene name | gltA |
| Synonyms | |
| Essential | no |
| Product | glutamate synthase (large subunit) |
| Function | glutamate biosynthesis |
| Gene expression levels in SubtiExpress: gltA | |
| Interactions involving this protein in SubtInteract: GltA | |
| Metabolic function and regulation of this protein in SubtiPathways: gltA | |
| MW, pI | 168 kDa, 5.47 |
| Gene length, protein length | 4560 bp, 1520 amino acids |
| Immediate neighbours | gltB, gltC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 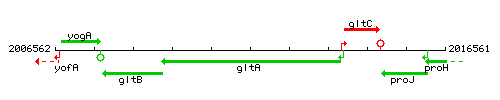
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed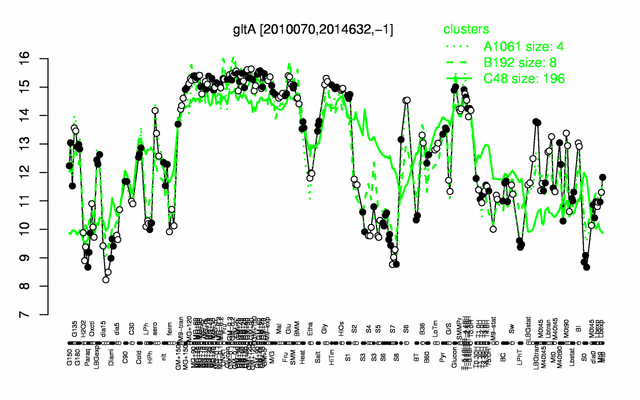
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, glutamate metabolism, membrane proteins, phosphoproteins
This gene is a member of the following regulons
GltC regulon, FsrA regulon, TnrA regulon, Efp-dependent proteins
The gene
Basic information
- Locus tag: BSU18450
Phenotypes of a mutant
auxotrophic for glutamate
Database entries
- BsubCyc: BSU18450
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 L-glutamate + NADP+ = L-glutamine + 2-oxoglutarate + NADPH (according to Swiss-Prot) 2 L-glutamate + NADP(+) <=> L-glutamine + 2-oxoglutarate + NADPH
- Protein family: glutamate synthase family (according to Swiss-Prot) glutamate synthase family
- Paralogous protein(s): YerD
Extended information on the protein
- Kinetic information:
- Domains:
- Glutamine amidotransferase type-2 domain (22-415)
- Nucleotide binding domain (1060-1112)
- Modification:
- phosphorylated on Arg-904 AND/OR Arg-914 PubMed
- Cofactor(s): 3Fe-4S, FAD, FMN
- Effectors of protein activity:
- Localization:
- membrane associated PubMed, cytoplasm
Database entries
- BsubCyc: BSU18450
- UniProt: P39812
- KEGG entry: [3]
- E.C. number: 1.4.1.13 3 1.4.1.13]
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
- translation is likely to require Efp due to the presence of several consecutive proline residues PubMed
Expression and regulation
- Regulation:
- expression activated by glucose (11 fold) (CcpA, GltC) PubMed
- repressed by arginine (GltC, RocG) PubMed
- expressed in the presence of ammonium PubMed
- repressed in the absence of good nitrogen sources (glutamine or ammonium) (TnrA) PubMed
- part of the iron sparing response, strong down-regulation in a fur mutant (Fur, FsrA) PubMed
- Regulatory mechanism:
- Additional information:
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
- translation is likely to require Efp due to the presence of several consecutive proline residues PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 2409 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 609 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 285 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 104 PubMed
Biological materials
- Mutant: GP807 (del gltAB::tet), GP222 (gltA under the control of p-xyl), available in Stülke lab
- Expression vector:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Jörg Stülke, University of Göttingen, Germany Homepage
Fabian Commichau University of Göttingen, Germany Homepage
Your additional remarks
References
Reviews
Original publications