Difference between revisions of "GlpP"
(→Expression and regulation) |
|||
| Line 90: | Line 90: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[yhxA]]-[[glpP]]'' {{PubMed|2127799}} |
* '''[[Sigma factor]]:''' | * '''[[Sigma factor]]:''' | ||
| Line 98: | Line 98: | ||
* '''Regulatory mechanism:''' | * '''Regulatory mechanism:''' | ||
| − | * '''Additional information:''' | + | * '''Additional information:''' |
=Biological materials = | =Biological materials = | ||
Revision as of 18:37, 4 September 2009
| Gene name | glpP |
| Synonyms | |
| Essential | no |
| Product | transcriptional antiterminator |
| Function | regulation of glycerol and glycerol-3-phosphate utilization |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism | |
| MW, pI | 21 kDa, 8.104 |
| Gene length, protein length | 576 bp, 192 aa |
| Immediate neighbours | yhxA, glpF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 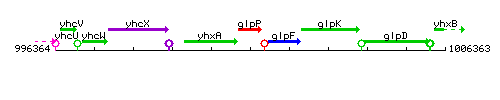
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU09270
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- UniProt: P30300
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Your additional remarks
References
Emmanuelle Darbon, Pascale Servant, Sandrine Poncet, Josef Deutscher
Antitermination by GlpP, catabolite repression via CcpA and inducer exclusion triggered by P-GlpK dephosphorylation control Bacillus subtilis glpFK expression.
Mol Microbiol: 2002, 43(4);1039-52
[PubMed:11929549]
[WorldCat.org]
[DOI]
(P p)
E Glatz, A Farewell, B Rutberg
The Bacillus subtilis glpD leader and antiterminator protein GlpP provide a target for glucose repression in Escherichia coli.
FEMS Microbiol Lett: 1998, 162(1);93-6
[PubMed:9595668]
[WorldCat.org]
[DOI]
(P p)
Elisabeth Glatz, Martin Persson, Blanka Rutberg
Antiterminator protein GlpP of Bacillus subtilis binds to glpD leader mRNA.
Microbiology (Reading): 1998, 144 ( Pt 2);449-456
[PubMed:9493382]
[WorldCat.org]
[DOI]
(P p)
E Glatz, R P Nilsson, L Rutberg, B Rutberg
A dual role for the Bacillus subtilis glpD leader and the GlpP protein in the regulated expression of glpD: antitermination and control of mRNA stability.
Mol Microbiol: 1996, 19(2);319-28
[PubMed:8825777]
[WorldCat.org]
[DOI]
(P p)
L Beijer, R P Nilsson, C Holmberg, L Rutberg
The glpP and glpF genes of the glycerol regulon in Bacillus subtilis.
J Gen Microbiol: 1993, 139(2);349-59
[PubMed:8436953]
[WorldCat.org]
[DOI]
(P p)
C Holmberg, L Rutberg
An inverted repeat preceding the Bacillus subtilis glpD gene is a conditional terminator of transcription.
Mol Microbiol: 1992, 6(20);2931-8
[PubMed:1479885]
[WorldCat.org]
[DOI]
(P p)
C Holmberg, B Rutberg
Expression of the gene encoding glycerol-3-phosphate dehydrogenase (glpD) in Bacillus subtilis is controlled by antitermination.
Mol Microbiol: 1991, 5(12);2891-900
[PubMed:1809833]
[WorldCat.org]
[DOI]
(P p)
V Lindgren, L Rutberg
Genetic control of the glp system in Bacillus subtilis.
J Bacteriol: 1976, 127(3);1047-57
[PubMed:182672]
[WorldCat.org]
[DOI]
(P p)