Difference between revisions of "GlcT"
| Line 39: | Line 39: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
| Line 121: | Line 117: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=glcT_1456092_1456958_1 glcT] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=glcT_1456092_1456958_1 glcT] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
* '''Regulation:''' | * '''Regulation:''' | ||
| Line 168: | Line 164: | ||
<pubmed> 9663674 18086213 </pubmed> | <pubmed> 9663674 18086213 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | + | <pubmed>11902727 9765562 12437213 10543968 17074746 15155854 14527945 , 19684596 22722928 20939030 22750856</pubmed> | |
| − | <pubmed>11902727 9765562 12437213 10543968 17074746 15155854 14527945 , 19684596 22722928 20939030 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 16:23, 27 May 2013
- Description: transcriptional antiterminator , controls expression of the ptsG-ptsH-ptsI operon
| Gene name | glcT |
| Synonyms | ykwA |
| Essential | no |
| Product | transcriptional antiterminator of the ptsG-ptsH-ptsI operon |
| Function | control of glucose uptake |
| Gene expression levels in SubtiExpress: glcT | |
| Interactions involving this protein in SubtInteract: GlcT | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 33,0 kDa, 7.01 |
| Gene length, protein length | 855 bp, 285 amino acids |
| Immediate neighbours | ykvZ, ptsG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed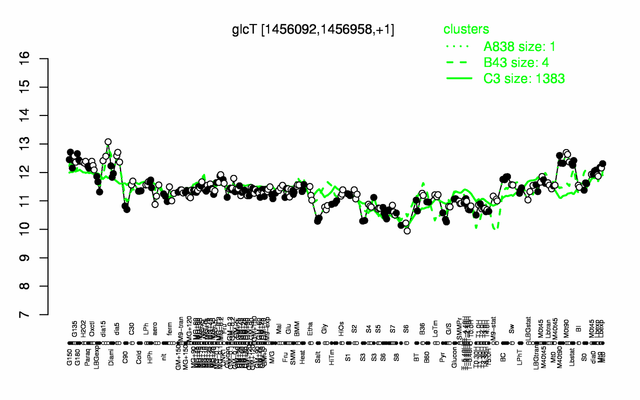
| |
Contents
Categories containing this gene/protein
carbon core metabolism, transcription factors and their control, RNA binding regulators, phosphoproteins
This gene is a member of the following regulons
The GlcT regulon: ptsG-ptsH-ptsI
The gene
Basic information
- Locus tag: BSU13880
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription antiterminator , RNA-binding protein, binds the ptsG RAT sequence
- Protein family: transcription antiterminator of the BglG/ SacY family
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation (His104)
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: O31691
- KEGG entry: [2]
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: available in Stülke lab:
- Expression vector:
- pGP124 (full length, in pWH844), available in Stülke lab
- pGP114 (amino acids 1-60, RNA-binding domain, in pWH844), available in Stülke lab
- pGP230 (amino acids 1-60, RNA-binding domain with thrombin cleavage site, in pWH844), available in Stülke lab
- pGP164 (both PRDs, in pWH844), in addition diverse expression vectors for phosphorylation site mutants and for RBD mutants (all in pWH844), available in Stülke lab
- pGP424 (PRDI, in pWH844), available in Stülke lab
- pGP425 (PRDII, in pWH844), available in Stülke lab
- pGP442 (PRDI, in pGP570, with thrombin cleavage site), available in Stülke lab
- pGP443 (PRDII, in pGP570, with thrombin cleavage site), available in Stülke lab
- pGP575 (amino acids 1-60, RNA-binding domain with Strep-tag, in pGP574), available in Stülke lab
- lacZ fusion:
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
Reviews
Fabian M Commichau, Jörg Stülke
Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression.
Mol Microbiol: 2008, 67(4);692-702
[PubMed:18086213]
[WorldCat.org]
[DOI]
(P p)
J Stülke, M Arnaud, G Rapoport, I Martin-Verstraete
PRD--a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria.
Mol Microbiol: 1998, 28(5);865-74
[PubMed:9663674]
[WorldCat.org]
[DOI]
(P p)
Original publications
Sebastian Himmel, Christian Grosse, Sebastian Wolff, Claudia Schwiegk, Stefan Becker
Structure of the RBD-PRDI fragment of the antiterminator protein GlcT.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2012, 68(Pt 7);751-6
[PubMed:22750856]
[WorldCat.org]
[DOI]
(I p)
Sebastian Himmel, Christopher P Zschiedrich, Stefan Becker, He-Hsuan Hsiao, Sebastian Wolff, Christine Diethmaier, Henning Urlaub, Donghan Lee, Christian Griesinger, Jörg Stülke
Determinants of interaction specificity of the Bacillus subtilis GlcT antitermination protein: functionality and phosphorylation specificity depend on the arrangement of the regulatory domains.
J Biol Chem: 2012, 287(33);27731-42
[PubMed:22722928]
[WorldCat.org]
[DOI]
(I p)
Sebastian Himmel, Sebastian Wolff, Stefan Becker, Donghan Lee, Christian Griesinger
Detection and identification of protein-phosphorylation sites in histidines through HNP correlation patterns.
Angew Chem Int Ed Engl: 2010, 49(47);8971-4
[PubMed:20939030]
[WorldCat.org]
[DOI]
(I p)
Dayté D Rodríguez, Christian Grosse, Sebastian Himmel, César González, Iñaki M de Ilarduya, Stefan Becker, George M Sheldrick, Isabel Usón
Crystallographic ab initio protein structure solution below atomic resolution.
Nat Methods: 2009, 6(9);651-3
[PubMed:19684596]
[WorldCat.org]
[DOI]
(I p)
Oliver Schilling, Christina Herzberg, Tina Hertrich, Hanna Vörsmann, Dirk Jessen, Sebastian Hübner, Fritz Titgemeyer, Jörg Stülke
Keeping signals straight in transcription regulation: specificity determinants for the interaction of a family of conserved bacterial RNA-protein couples.
Nucleic Acids Res: 2006, 34(21);6102-15
[PubMed:17074746]
[WorldCat.org]
[DOI]
(I p)
Oliver Schilling, Ines Langbein, Michael Müller, Matthias H Schmalisch, Jörg Stülke
A protein-dependent riboswitch controlling ptsGHI operon expression in Bacillus subtilis: RNA structure rather than sequence provides interaction specificity.
Nucleic Acids Res: 2004, 32(9);2853-64
[PubMed:15155854]
[WorldCat.org]
[DOI]
(I e)
Matthias H Schmalisch, Steffi Bachem, Jörg Stülke
Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation. Elucidation of the phosphorylation chain leading to inactivation of GlcT.
J Biol Chem: 2003, 278(51);51108-15
[PubMed:14527945]
[WorldCat.org]
[DOI]
(P p)
David B Greenberg, Jorg Stülke, Milton H Saier
Domain analysis of transcriptional regulators bearing PTS regulatory domains.
Res Microbiol: 2002, 153(8);519-26
[PubMed:12437213]
[WorldCat.org]
[DOI]
(P p)
I Langbein, S Bachem, J Stülke
Specific interaction of the RNA-binding domain of the bacillus subtilis transcriptional antiterminator GlcT with its RNA target, RAT.
J Mol Biol: 1999, 293(4);795-805
[PubMed:10543968]
[WorldCat.org]
[DOI]
(P p)
S Bachem, J Stülke
Regulation of the Bacillus subtilis GlcT antiterminator protein by components of the phosphotransferase system.
J Bacteriol: 1998, 180(20);5319-26
[PubMed:9765562]
[WorldCat.org]
[DOI]
(P p)
J Stülke, I Martin-Verstraete, M Zagorec, M Rose, A Klier, G Rapoport
Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT.
Mol Microbiol: 1997, 25(1);65-78
[PubMed:11902727]
[WorldCat.org]
[DOI]
(P p)