GgaA
- Description: galactosamine-containing minor teichoic acid biosynthesis
| Gene name | ggaA |
| Synonyms | |
| Essential | no |
| Product | unknown |
| Function | biosynthesis of teichoic acid |
| Gene expression levels in SubtiExpress: ggaA | |
| Metabolic function and regulation of this protein in SubtiPathways: GgaA | |
| MW, pI | 52 kDa, 9.055 |
| Gene length, protein length | 1338 bp, 446 aa |
| Immediate neighbours | ggaB, yvzI |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 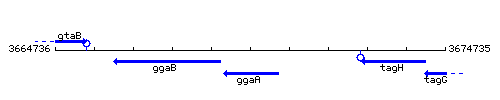
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed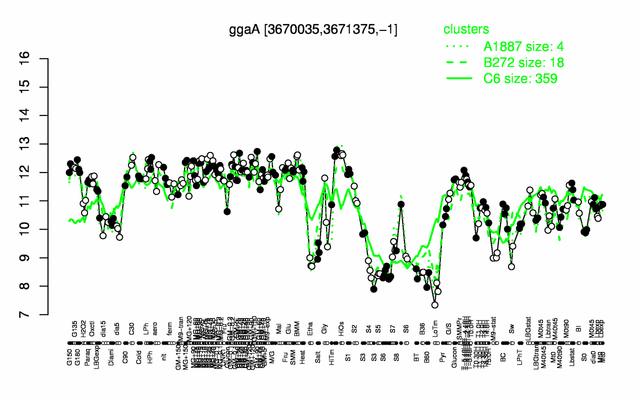
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis of cell wall components, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35690
Phenotypes of a mutant
Database entries
- BsubCyc: BSU35690
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: glycosyltransferase 2 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-85 PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU35690
- Structure:
- UniProt: P46917
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
- An antisense RNA is predicted for ggaA PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Letal I Salzberg, Eric Botella, Karsten Hokamp, Haike Antelmann, Sandra Maaß, Dörte Becher, David Noone, Kevin M Devine
Genome-wide analysis of phosphorylated PhoP binding to chromosomal DNA reveals several novel features of the PhoPR-mediated phosphate limitation response in Bacillus subtilis.
J Bacteriol: 2015, 197(8);1492-506
[PubMed:25666134]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
Pierre-Philippe Freymond, Vladimir Lazarevic, Blazenka Soldo, Dimitri Karamata
Poly(glucosyl-N-acetylgalactosamine 1-phosphate), a wall teichoic acid of Bacillus subtilis 168: its biosynthetic pathway and mode of attachment to peptidoglycan.
Microbiology (Reading): 2006, 152(Pt 6);1709-1718
[PubMed:16735734]
[WorldCat.org]
[DOI]
(P p)