Difference between revisions of "GabR"
(→References) |
|||
| Line 145: | Line 145: | ||
=References= | =References= | ||
| − | <pubmed> 11756427 15223311,12123465, 24127574 22020104 </pubmed> | + | <pubmed> 11756427 15223311,12123465, 24127574 22020104 25388514 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 06:11, 14 November 2014
- Description: transcription activator of gabT-gabD, repressor of gabR (MocR/ GabR family)
| Gene name | gabR |
| Synonyms | ycnF |
| Essential | no |
| Product | transcription regulator |
| Function | regulation of gamma-amino butyric acid utilization |
| Gene expression levels in SubtiExpress: gabR | |
| MW, pI | 54 kDa, 6.709 |
| Gene length, protein length | 1437 bp, 479 aa |
| Immediate neighbours | yczG, gabT |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 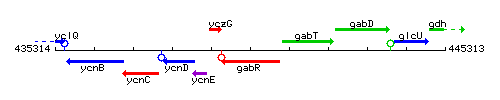
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
utilization of amino acids, transcription factors and their control
This gene is a member of the following regulons
The GabR regulon:
The gene
Basic information
- Locus tag: BSU03890
Phenotypes of a mutant
Database entries
- BsubCyc: BSU03890
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: MocR/ GabR family PubMed
- Paralogous protein(s): none, but there are 7 members of the MocR/ GabR family in B. subtilis
Extended information on the protein
- Kinetic information:
- Domains:
- N-terminal DNA-binding helix-turn-helix motif (corresponding to domains of the GntR family) PubMed
- C-terminal domain is homologous to PLP-binding large domain of aminotransferases PubMed
- Modification:
- Effectors of protein activity:
- Interactions:
- forms head-to-tail domain-swapped dimers PubMed
Database entries
- BsubCyc: BSU03890
- UniProt: P94426
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- GabR: negative autoregulation PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Your additional remarks
References
Keita Okuda, Shiro Kato, Tomokazu Ito, Shunsuke Shiraki, Yumiko Kawase, Masaru Goto, Susumu Kawashima, Hisashi Hemmi, Harumi Fukada, Tohru Yoshimura
Role of the aminotransferase domain in Bacillus subtilis GabR, a pyridoxal 5'-phosphate-dependent transcriptional regulator.
Mol Microbiol: 2015, 95(2);245-57
[PubMed:25388514]
[WorldCat.org]
[DOI]
(I p)
Raji Edayathumangalam, Rui Wu, Roman Garcia, Yuguang Wang, Wei Wang, Cheryl A Kreinbring, Alicia Bach, Jingling Liao, Todd A Stone, Thomas C Terwilliger, Quyen Q Hoang, Boris R Belitsky, Gregory A Petsko, Dagmar Ringe, Dali Liu
Crystal structure of Bacillus subtilis GabR, an autorepressor and transcriptional activator of gabT.
Proc Natl Acad Sci U S A: 2013, 110(44);17820-5
[PubMed:24127574]
[WorldCat.org]
[DOI]
(I p)
E Bramucci, T Milano, S Pascarella
Genomic distribution and heterogeneity of MocR-like transcriptional factors containing a domain belonging to the superfamily of the pyridoxal-5'-phosphate dependent enzymes of fold type I.
Biochem Biophys Res Commun: 2011, 415(1);88-93
[PubMed:22020104]
[WorldCat.org]
[DOI]
(I p)
Boris R Belitsky
Bacillus subtilis GabR, a protein with DNA-binding and aminotransferase domains, is a PLP-dependent transcriptional regulator.
J Mol Biol: 2004, 340(4);655-64
[PubMed:15223311]
[WorldCat.org]
[DOI]
(P p)
Boris R Belitsky, Abraham L Sonenshein
GabR, a member of a novel protein family, regulates the utilization of gamma-aminobutyrate in Bacillus subtilis.
Mol Microbiol: 2002, 45(2);569-83
[PubMed:12123465]
[WorldCat.org]
[DOI]
(P p)
Sébastien Rigali, Adeline Derouaux, Fabrizio Giannotta, Jean Dusart
Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies.
J Biol Chem: 2002, 277(15);12507-15
[PubMed:11756427]
[WorldCat.org]
[DOI]
(P p)