Difference between revisions of "FbaA"
| Line 36: | Line 36: | ||
=== Basic information === | === Basic information === | ||
| − | * '''Locus tag:''' | + | * '''Locus tag:''' BSU37120 |
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
| Line 83: | Line 83: | ||
* '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P13243 P13243] | * '''Swiss prot entry:''' [http://www.uniprot.org/uniprot/P13243 P13243] | ||
| − | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+ | + | * '''KEGG entry:''' [http://www.genome.jp/dbget-bin/www_bget?bsu+BSU37120] |
* '''E.C. number:''' [http://www.expasy.org/enzyme/4.1.2.13 4.1.2.13] 3 4.1.2.13] | * '''E.C. number:''' [http://www.expasy.org/enzyme/4.1.2.13 4.1.2.13] 3 4.1.2.13] | ||
Revision as of 09:27, 3 June 2009
- Description: fructose 1,6-bisphosphate aldolase, glycolytic/ gluconeogenic enzyme
| Gene name | fbaA |
| Synonyms | fba, fba1, tsr |
| Essential | yes |
| Product | fructose-1,6-bisphosphate aldolase |
| Function | enzyme in glycolysis/ gluconeogenesis |
| MW, pI | 30,2 kDa, 5.03 |
| Gene length, protein length | 855 bp, 285 amino acids |
| Immediate neighbours | spo0F, ywjH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 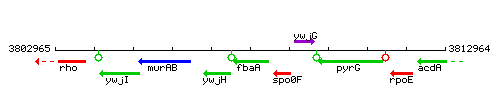
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU37120
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: D-fructose 1,6-bisphosphate = glycerone phosphate + D-glyceraldehyde 3-phosphate (according to Swiss-Prot) D-fructose 1,6-bisphosphate = dihydroxyacetone phosphate + D-glyceraldehyde 3-phosphate
- Protein family: class II fructose-bisphosphate aldolase family (according to Swiss-Prot) class II fructose-bisphosphate aldolase family
- Paralogous protein(s): FbaB
Extended information on the protein
- Kinetic information:
- Domains:
- 2 x Dihydroxyacetone phosphate binding domain (210–212), (231–234)
- Modification: phosphorylation on Thr-212 AND Thr-234 PubMed
- Cofactor(s): 2 x zinc ion
- Effectors of protein activity: inhibited by alpha-keto acids PubMed
- Interactions:
- Localization:
Database entries
- Structure:
- Swiss prot entry: P13243
- KEGG entry: [3]
- E.C. number: 4.1.2.13 3 4.1.2.13]
Additional information
Binds 2 zinc ions per subunit. One is catalytic and the other provides a structural contribution
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector: pGP395 (N-terminal His-tag, in pWH844), pGP88 (N-terminal Strep-tag, for SPINE, expression in B. subtilis, in pGP380)
- lacZ fusion: pGP601 (in pAC6)
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
- Trach K, Chapman JW & Piggot P (1988) Complete sequence and transcriptional analysis of the spo0F region of the Bacillus subtilis chromosome J Bacteriol. 170: 4194-4208. PubMed
- Ludwig H, Homuth G & Schmalisch M (2001) Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon Mol Microbiol. 41: 409-422. PubMed
- Macek et al. (2007) The serine/ threonine/ tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6: 697-707 PubMed