Difference between revisions of "FabL"
| Line 129: | Line 129: | ||
** number of protein molecules per cell (minimal medium with glucose and ammonium): 206 {{PubMed|24696501}} | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 206 {{PubMed|24696501}} | ||
** number of protein molecules per cell (complex medium with amino acids, without glucose): 750 {{PubMed|24696501}} | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 750 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1540 {{PubMed|21395229}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 1009 {{PubMed|21395229}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1634 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 14:06, 17 April 2014
- Description: enoyl-acyl carrier protein reductase
| Gene name | fabL |
| Synonyms | yfhR, ygaA |
| Essential | no |
| Product | enoyl-acyl carrier protein reductase |
| Function | fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: fabL | |
| Metabolic function and regulation of this protein in SubtiPathways: fabL | |
| MW, pI | 27 kDa, 5.967 |
| Gene length, protein length | 750 bp, 250 aa |
| Immediate neighbours | yfhS, sspE |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 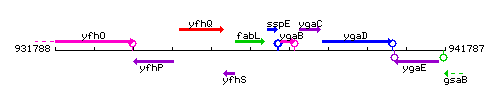
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed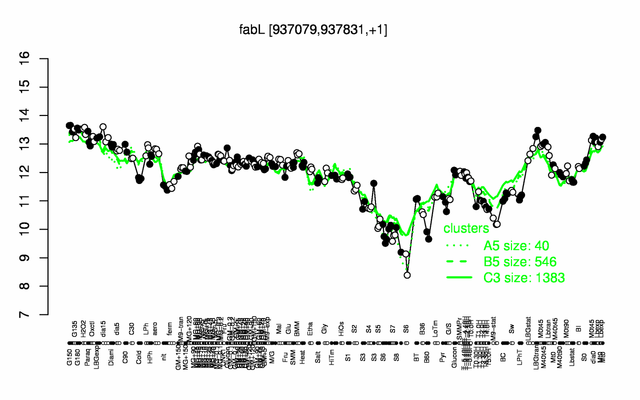
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, sporulation proteins
This gene is a member of the following regulons
SigG regulon, SpoVT regulon, YfhP regulon
The gene
Basic information
- Locus tag: BSU08650
Phenotypes of a mutant
Database entries
- BsubCyc: BSU08650
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU08650
- UniProt: P71079
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 206 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 750 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1540 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 1009 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1634 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Yasutaro Fujita, Hiroshi Matsuoka, Kazutake Hirooka
Regulation of fatty acid metabolism in bacteria.
Mol Microbiol: 2007, 66(4);829-39
[PubMed:17919287]
[WorldCat.org]
[DOI]
(P p)
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Kook-Han Kim, Byung Hak Ha, Su Jin Kim, Seung Kon Hong, Kwang Yeon Hwang, Eunice Eunkyeong Kim
Crystal structures of Enoyl-ACP reductases I (FabI) and III (FabL) from B. subtilis.
J Mol Biol: 2011, 406(3);403-15
[PubMed:21185310]
[WorldCat.org]
[DOI]
(I p)
Helena B Thomaides, Ella J Davison, Lisa Burston, Hazel Johnson, David R Brown, Alison C Hunt, Jeffery Errington, Lloyd Czaplewski
Essential bacterial functions encoded by gene pairs.
J Bacteriol: 2007, 189(2);591-602
[PubMed:17114254]
[WorldCat.org]
[DOI]
(P p)
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
R J Heath, N Su, C K Murphy, C O Rock
The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis.
J Biol Chem: 2000, 275(51);40128-33
[PubMed:11007778]
[WorldCat.org]
[DOI]
(P p)
Hiroki Yamamoto, Masao Mori, Junichi Sekiguchi
Transcription of genes near the sspE locus of the Bacillus subtilis genome.
Microbiology (Reading): 1999, 145 ( Pt 8);2171-2180
[PubMed:10463184]
[WorldCat.org]
[DOI]
(P p)
I Bagyan, J Hobot, S Cutting
A compartmentalized regulator of developmental gene expression in Bacillus subtilis.
J Bacteriol: 1996, 178(15);4500-7
[PubMed:8755877]
[WorldCat.org]
[DOI]
(P p)