Difference between revisions of "FabG"
| Line 14: | Line 14: | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || fatty acid biosynthesis | |style="background:#ABCDEF;" align="center"|'''Function''' || fatty acid biosynthesis | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http:// | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://subtiwiki.uni-goettingen.de/apps/expression/ ''Subti''Express]''': [http://subtiwiki.uni-goettingen.de/apps/expression/expression.php?search=BSU15910 fabG] |
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/fatty_acid_synthesis.html Lipid synthesis]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/fatty_acid_synthesis.html Lipid synthesis]''' | ||
| Line 24: | Line 24: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[fabD]]'', ''[[acpA]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[fabD]]'', ''[[acpA]]'' | ||
|- | |- | ||
| − | | | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU15910 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU15910 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU15910 Advanced_DNA] |
|- | |- | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:fabG_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:fabG_context.gif]] | ||
Revision as of 13:48, 13 May 2013
- Description: beta-ketoacyl-acyl carrier protein reductase
| Gene name | fabG |
| Synonyms | ylpF |
| Essential | yes PubMed |
| Product | beta-ketoacyl-acyl carrier protein reductase |
| Function | fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: fabG | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 26 kDa, 8.091 |
| Gene length, protein length | 738 bp, 246 aa |
| Immediate neighbours | fabD, acpA |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 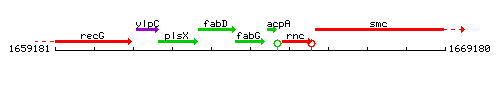
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed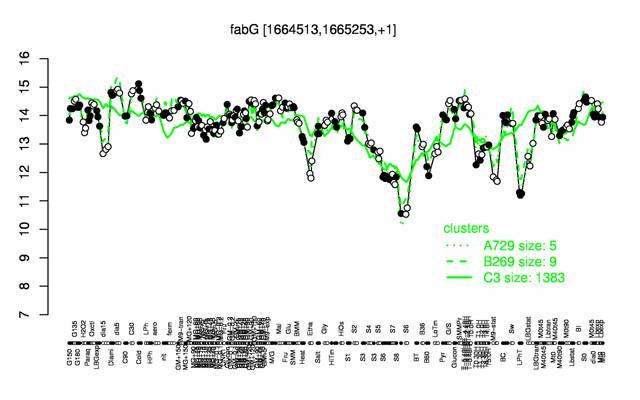
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15910
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: (3R)-3-hydroxyacyl-[acyl-carrier-protein] + NADP+ = 3-oxoacyl-[acyl-carrier-protein] + NADPH (according to Swiss-Prot)
- Protein family: short-chain dehydrogenases/reductases (SDR) family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- UniProt: P51831
- KEGG entry: [3]
- E.C. number: 1.1.1.100
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Yasutaro Fujita, Hiroshi Matsuoka, Kazutake Hirooka
Regulation of fatty acid metabolism in bacteria.
Mol Microbiol: 2007, 66(4);829-39
[PubMed:17919287]
[WorldCat.org]
[DOI]
(P p)
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Mariano A Martinez, Diego de Mendoza, Gustavo E Schujman
Transcriptional and functional characterization of the gene encoding acyl carrier protein in Bacillus subtilis.
Microbiology (Reading): 2010, 156(Pt 2);484-495
[PubMed:19850612]
[WorldCat.org]
[DOI]
(I p)
Gustavo E Schujman, Marcelo Guerin, Alejandro Buschiazzo, Francis Schaeffer, Leticia I Llarrull, Georgina Reh, Alejandro J Vila, Pedro M Alzari, Diego de Mendoza
Structural basis of lipid biosynthesis regulation in Gram-positive bacteria.
EMBO J: 2006, 25(17);4074-83
[PubMed:16932747]
[WorldCat.org]
[DOI]
(P p)
Natalia Comella, Alan D Grossman
Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis.
Mol Microbiol: 2005, 57(4);1159-74
[PubMed:16091051]
[WorldCat.org]
[DOI]
(P p)
Gustavo E Schujman, Luciana Paoletti, Alan D Grossman, Diego de Mendoza
FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis.
Dev Cell: 2003, 4(5);663-72
[PubMed:12737802]
[WorldCat.org]
[DOI]
(P p)
A C Price, Y M Zhang, C O Rock, S W White
Structure of beta-ketoacyl-[acyl carrier protein] reductase from Escherichia coli: negative cooperativity and its structural basis.
Biochemistry: 2001, 40(43);12772-81
[PubMed:11669613]
[WorldCat.org]
[DOI]
(P p)