Difference between revisions of "DnaX"
| Line 24: | Line 24: | ||
|style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[scr]]'', ''[[yaaK]]'' | |style="background:#ABCDEF;" align="center"|'''Immediate neighbours''' || ''[[scr]]'', ''[[yaaK]]'' | ||
|- | |- | ||
| − | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU00190 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU00190 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU00190 | + | |style="background:#FAF8CC;" align="center"|'''Sequences'''||[http://bsubcyc.org/BSUB/sequence-aa?type=GENE&object=BSU00190 Protein] [http://bsubcyc.org/BSUB/sequence?type=GENE&object=BSU00190 DNA] [http://bsubcyc.org/BSUB/seq-selector?chromosome=CHROM-1&object=BSU00190 DNA_with_flanks] |
|- | |- | ||
|- | |- | ||
Revision as of 09:07, 14 May 2013
- Description: DNA polymerase III (gamma and tau subunits) part of the clamp-loader complex and the replisome
| Gene name | dnaX |
| Synonyms | dnaH, dna-8132 |
| Essential | yes PubMed |
| Product | DNA polymerase III (gamma and tau subunits) |
| Function | DNA replication |
| Gene expression levels in SubtiExpress: dnaX | |
| Interactions involving this protein in SubtInteract: DnaX | |
| MW, pI | 62 kDa, 5.488 |
| Gene length, protein length | 1689 bp, 563 aa |
| Immediate neighbours | scr, yaaK |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 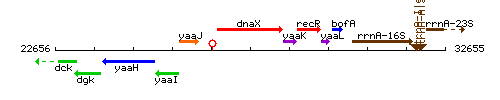
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
DNA replication, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU00190
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1) (according to Swiss-Prot)
- required for bacteriophage SPP1 replication PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P09122
- KEGG entry: [2]
- E.C. number: 2.7.7.7
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
José P Afonso, Kiran Chintakayala, Chatrudee Suwannachart, Svetlana Sedelnikova, Kevin Giles, John B Hoyes, Panos Soultanas, John B Rafferty, Neil J Oldham
Insights into the structure and assembly of the Bacillus subtilis clamp-loader complex and its interaction with the replicative helicase.
Nucleic Acids Res: 2013, 41(9);5115-26
[PubMed:23525462]
[WorldCat.org]
[DOI]
(I p)
Elena M Seco, John C Zinder, Carol M Manhart, Ambra Lo Piano, Charles S McHenry, Silvia Ayora
Bacteriophage SPP1 DNA replication strategies promote viral and disable host replication in vitro.
Nucleic Acids Res: 2013, 41(3);1711-21
[PubMed:23268446]
[WorldCat.org]
[DOI]
(I p)
Andrew D Klocko, Jeremy W Schroeder, Brian W Walsh, Justin S Lenhart, Margery L Evans, Lyle A Simmons
Mismatch repair causes the dynamic release of an essential DNA polymerase from the replication fork.
Mol Microbiol: 2011, 82(3);648-63
[PubMed:21958350]
[WorldCat.org]
[DOI]
(I p)
Glenn M Sanders, H Garry Dallmann, Charles S McHenry
Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases.
Mol Cell: 2010, 37(2);273-81
[PubMed:20122408]
[WorldCat.org]
[DOI]
(I p)
Kiran Chintakayala, Cristina Machón, Anna Haroniti, Marilyn A Larson, Steven H Hinrichs, Mark A Griep, Panos Soultanas
Allosteric regulation of the primase (DnaG) activity by the clamp-loader (tau) in vitro.
Mol Microbiol: 2009, 72(2);537-49
[PubMed:19415803]
[WorldCat.org]
[DOI]
(I p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Anna Haroniti, Christopher Anderson, Zara Doddridge, Laurence Gardiner, Clive J Roberts, Stephanie Allen, Panos Soultanas
The clamp-loader-helicase interaction in Bacillus. Atomic force microscopy reveals the structural organisation of the DnaB-tau complex in Bacillus.
J Mol Biol: 2004, 336(2);381-93
[PubMed:14757052]
[WorldCat.org]
[DOI]
(P p)
A Haroniti, R Till, M C M Smith, P Soultanas
Clamp-loader-helicase interaction in Bacillus. Leucine 381 is critical for pentamerization and helicase binding of the Bacillus tau protein.
Biochemistry: 2003, 42(37);10955-64
[PubMed:12974630]
[WorldCat.org]
[DOI]
(P p)
María I Martínez-Jiménez, Pablo Mesa, Juan C Alonso
Bacillus subtilis tau subunit of DNA polymerase III interacts with bacteriophage SPP1 replicative DNA helicase G40P.
Nucleic Acids Res: 2002, 30(23);5056-64
[PubMed:12466528]
[WorldCat.org]
[DOI]
(I p)
J C Struck, J C Alonso, H Y Toschka, V A Erdmann
The Bacillus subtilis small cytoplasmic RNA gene and 'dnaX' map near the chromosomal replication origin.
Mol Gen Genet: 1990, 222(2-3);470-2
[PubMed:1703271]
[WorldCat.org]
[DOI]
(P p)