Difference between revisions of "DeaD"
| Line 28: | Line 28: | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:deaD_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:deaD_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=deaD_4015987_4017426_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:deaD_expression.png|500px]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | |||
<br/><br/> | <br/><br/> | ||
Revision as of 12:22, 23 April 2012
- Description: DEAD-box RNA helicase
| Gene name | deaD |
| Synonyms | yxiN |
| Essential | no |
| Product | DEAD-box RNA helicase |
| Function | RNA helicase |
| Interactions involving this protein in SubtInteract: DeaD | |
| MW, pI | 53 kDa, 7.706 |
| Gene length, protein length | 1437 bp, 479 aa |
| Immediate neighbours | yxiO, yxiM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 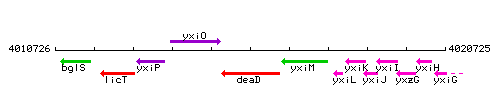
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed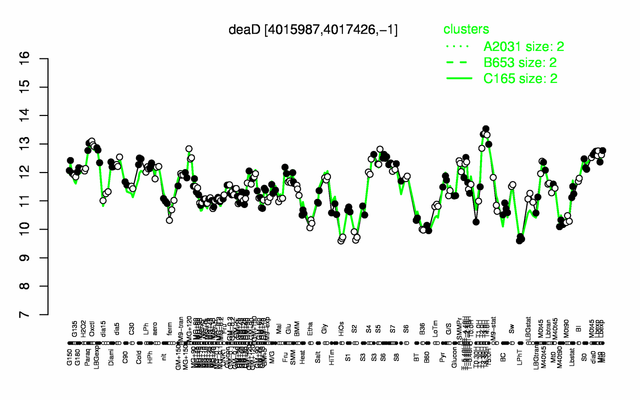
| |
Contents
Categories containing this gene/protein
DEAD-box RNA helicases, translation
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU39110
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: RNA helicase
- Protein family: DEAD-box RNA helicase
Extended information on the protein
- Kinetic information:
- Domains:
- Helicase domain 1: AS 1-203
- Linker region: AS 204-212
- Helicase domain 2: AS 207-368
- RNA-binding domain: AS 404-479
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 2G0C (RNA-binding domain, AA 404-479), 2HJV (second domain, AA 207-368); 3MOJ (RNA binding domain complexed with a fragment of 23S ribosomal RNA) PubMed
- UniProt: P42305
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP1121 (tet), available in the Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Dagmar Klostermeier, Biozentrum Basel, Switzerland homepage
Your additional remarks
References
Anne R Karow, Dagmar Klostermeier
A structural model for the DEAD box helicase YxiN in solution: localization of the RNA binding domain.
J Mol Biol: 2010, 402(4);629-37
[PubMed:20691700]
[WorldCat.org]
[DOI]
(I p)
John W Hardin, Yao Xiong Hu, David B McKay
Structure of the RNA binding domain of a DEAD-box helicase bound to its ribosomal RNA target reveals a novel mode of recognition by an RNA recognition motif.
J Mol Biol: 2010, 402(2);412-27
[PubMed:20673833]
[WorldCat.org]
[DOI]
(I p)
Martin Lehnik-Habrink, Henrike Pförtner, Leonie Rempeters, Nico Pietack, Christina Herzberg, Jörg Stülke
The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multiprotein complex.
Mol Microbiol: 2010, 77(4);958-71
[PubMed:20572937]
[WorldCat.org]
[DOI]
(I p)
Regula Aregger, Dagmar Klostermeier
The DEAD box helicase YxiN maintains a closed conformation during ATP hydrolysis.
Biochemistry: 2009, 48(45);10679-81
[PubMed:19839642]
[WorldCat.org]
[DOI]
(I p)
Anne R Karow, Dagmar Klostermeier
A conformational change in the helicase core is necessary but not sufficient for RNA unwinding by the DEAD box helicase YxiN.
Nucleic Acids Res: 2009, 37(13);4464-71
[PubMed:19474341]
[WorldCat.org]
[DOI]
(I p)
Bettina Theissen, Anne R Karow, Jürgen Köhler, Airat Gubaev, Dagmar Klostermeier
Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase.
Proc Natl Acad Sci U S A: 2008, 105(2);548-53
[PubMed:18184816]
[WorldCat.org]
[DOI]
(I p)
Shuying Wang, Michael T Overgaard, YaoXiong Hu, David B McKay
The Bacillus subtilis RNA helicase YxiN is distended in solution.
Biophys J: 2008, 94(1);L01-3
[PubMed:17951299]
[WorldCat.org]
[DOI]
(I p)
Anne R Karow, Bettina Theissen, Dagmar Klostermeier
Authentic interdomain communication in an RNA helicase reconstituted by expressed protein ligation of two helicase domains.
FEBS J: 2007, 274(2);463-73
[PubMed:17229151]
[WorldCat.org]
[DOI]
(P p)
Jonathan M Caruthers, YaoXiong Hu, David B McKay
Structure of the second domain of the Bacillus subtilis DEAD-box RNA helicase YxiN.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2006, 62(Pt 12);1191-5
[PubMed:17142894]
[WorldCat.org]
[DOI]
(I p)
Shuying Wang, Yaoxiong Hu, Michael T Overgaard, Fedor V Karginov, Olke C Uhlenbeck, David B McKay
The domain of the Bacillus subtilis DEAD-box helicase YxiN that is responsible for specific binding of 23S rRNA has an RNA recognition motif fold.
RNA: 2006, 12(6);959-67
[PubMed:16611943]
[WorldCat.org]
[DOI]
(P p)
Fedor V Karginov, Jonathan M Caruthers, YaoXiong Hu, David B McKay, Olke C Uhlenbeck
YxiN is a modular protein combining a DEx(D/H) core and a specific RNA-binding domain.
J Biol Chem: 2005, 280(42);35499-505
[PubMed:16118224]
[WorldCat.org]
[DOI]
(P p)
Karl Kossen, Fedor V Karginov, Olke C Uhlenbeck
The carboxy-terminal domain of the DExDH protein YxiN is sufficient to confer specificity for 23S rRNA.
J Mol Biol: 2002, 324(4);625-36
[PubMed:12460566]
[WorldCat.org]
[DOI]
(P p)
K Kossen, O C Uhlenbeck
Cloning and biochemical characterization of Bacillus subtilis YxiN, a DEAD protein specifically activated by 23S rRNA: delineation of a novel sub-family of bacterial DEAD proteins.
Nucleic Acids Res: 1999, 27(19);3811-20
[PubMed:10481020]
[WorldCat.org]
[DOI]
(I p)