Difference between revisions of "DapG"
| Line 39: | Line 39: | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
| − | + | <br/><br/> | |
| − | |||
| − | |||
| − | |||
| − | |||
= [[Categories]] containing this gene/protein = | = [[Categories]] containing this gene/protein = | ||
| + | {{SubtiWiki category|[[cell wall synthesis]]}}, | ||
{{SubtiWiki category|[[biosynthesis/ acquisition of amino acids]]}}, | {{SubtiWiki category|[[biosynthesis/ acquisition of amino acids]]}}, | ||
| + | {{SubtiWiki category|[[Biosynthesis of cell wall components]]}}, | ||
{{SubtiWiki category|[[sporulation proteins]]}} | {{SubtiWiki category|[[sporulation proteins]]}} | ||
| Line 67: | Line 65: | ||
=== Additional information=== | === Additional information=== | ||
| − | |||
| − | |||
| − | |||
=The protein= | =The protein= | ||
| Line 85: | Line 80: | ||
* '''Kinetic information:''' | * '''Kinetic information:''' | ||
| − | * '''Domains:''' | + | * '''[[Domains]]:''' |
* '''Modification:''' | * '''Modification:''' | ||
| − | * ''' | + | * '''[[Cofactors]]:''' |
* '''Effectors of protein activity:''' inhibited by diaminopimelic acid {{PubMed|2152900}} | * '''Effectors of protein activity:''' inhibited by diaminopimelic acid {{PubMed|2152900}} | ||
| Line 117: | Line 112: | ||
* '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dapG_1747123_1748337_1 dapG] {{PubMed|22383849}} | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=dapG_1747123_1748337_1 dapG] {{PubMed|22383849}} | ||
| − | * '''Sigma factor:''' | + | * '''[[Sigma factor]]:''' |
** ''[[spoVFA]]'': [[SigK]] {{PubMed|15699190,8098035}} | ** ''[[spoVFA]]'': [[SigK]] {{PubMed|15699190,8098035}} | ||
** ''[[asd]]'': [[SigA]] {{PubMed|8098035}} | ** ''[[asd]]'': [[SigA]] {{PubMed|8098035}} | ||
Revision as of 11:50, 5 January 2014
- Description: aspartokinase I (alpha and beta subunits)
| Gene name | dapG |
| Synonyms | lssD |
| Essential | no |
| Product | aspartokinase I (alpha and beta subunits) |
| Function | biosynthesis of lysine and peptidoglycan |
| Gene expression levels in SubtiExpress: dapG | |
| Metabolic function and regulation of this protein in SubtiPathways: Lys, Thr | |
| MW, pI | 42 kDa, 5.709 |
| Gene length, protein length | 1212 bp, 404 aa |
| Immediate neighbours | asd, dapA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 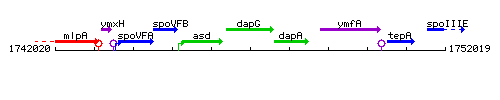
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed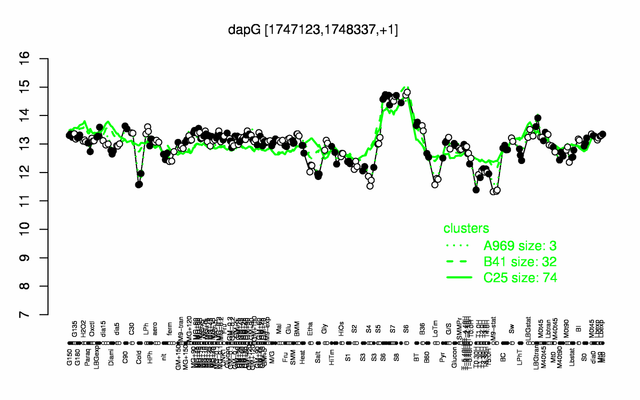
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis/ acquisition of amino acids, Biosynthesis of cell wall components, sporulation proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16760
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-aspartate = ADP + 4-phospho-L-aspartate (according to Swiss-Prot)
- Protein family: aspartokinase family (according to Swiss-Prot)
- Paralogous protein(s): LysC
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity: inhibited by diaminopimelic acid PubMed
Database entries
- Structure:
- UniProt: Q04795
- KEGG entry: [3]
- E.C. number: 2.7.2.4
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Chien-Chi Lo, Carol A Bonner, Gary Xie, Mark D'Souza, Roy A Jensen
Cohesion group approach for evolutionary analysis of aspartokinase, an enzyme that feeds a branched network of many biochemical pathways.
Microbiol Mol Biol Rev: 2009, 73(4);594-651
[PubMed:19946135]
[WorldCat.org]
[DOI]
(I p)
Original publications
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
R A Daniel, J Errington
Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis.
J Mol Biol: 1993, 232(2);468-83
[PubMed:8345520]
[WorldCat.org]
[DOI]
(P p)
N Y Chen, S Q Jiang, D A Klein, H Paulus
Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase.
J Biol Chem: 1993, 268(13);9448-65
[PubMed:8098035]
[WorldCat.org]
(P p)
L M Graves, R L Switzer
Aspartokinase III, a new isozyme in Bacillus subtilis 168.
J Bacteriol: 1990, 172(1);218-23
[PubMed:2152900]
[WorldCat.org]
[DOI]
(P p)