Difference between revisions of "DapG"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || biosynthesis of lysine and peptidoglycan | |style="background:#ABCDEF;" align="center"|'''Function''' || biosynthesis of lysine and peptidoglycan | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU16760 dapG] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/lys_threo.html Lys, Thr]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/lys_threo.html Lys, Thr]''' | ||
Revision as of 09:53, 7 August 2012
- Description: aspartokinase I (alpha and beta subunits)
| Gene name | dapG |
| Synonyms | lssD |
| Essential | no |
| Product | aspartokinase I (alpha and beta subunits) |
| Function | biosynthesis of lysine and peptidoglycan |
| Gene expression levels in SubtiExpress: dapG | |
| Metabolic function and regulation of this protein in SubtiPathways: Lys, Thr | |
| MW, pI | 42 kDa, 5.709 |
| Gene length, protein length | 1212 bp, 404 aa |
| Immediate neighbours | asd, dapA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 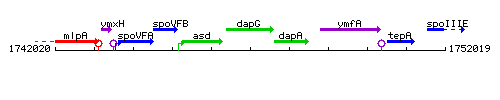
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, sporulation proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16760
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + L-aspartate = ADP + 4-phospho-L-aspartate (according to Swiss-Prot)
- Protein family: aspartokinase family (according to Swiss-Prot)
- Paralogous protein(s): LysC
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity: inhibited by diaminopimelic acid PubMed
Database entries
- Structure:
- UniProt: Q04795
- KEGG entry: [3]
- E.C. number: 2.7.2.4
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Chien-Chi Lo, Carol A Bonner, Gary Xie, Mark D'Souza, Roy A Jensen
Cohesion group approach for evolutionary analysis of aspartokinase, an enzyme that feeds a branched network of many biochemical pathways.
Microbiol Mol Biol Rev: 2009, 73(4);594-651
[PubMed:19946135]
[WorldCat.org]
[DOI]
(I p)
Original publications
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
R A Daniel, J Errington
Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis.
J Mol Biol: 1993, 232(2);468-83
[PubMed:8345520]
[WorldCat.org]
[DOI]
(P p)
N Y Chen, S Q Jiang, D A Klein, H Paulus
Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase.
J Biol Chem: 1993, 268(13);9448-65
[PubMed:8098035]
[WorldCat.org]
(P p)
L M Graves, R L Switzer
Aspartokinase III, a new isozyme in Bacillus subtilis 168.
J Bacteriol: 1990, 172(1);218-23
[PubMed:2152900]
[WorldCat.org]
[DOI]
(P p)