Difference between revisions of "CopZ"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || resistance to copper | |style="background:#ABCDEF;" align="center"|'''Function''' || resistance to copper | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://cellpublisher.gobics.de/subtinteract/startpage/start/ ''Subt''Interact]''': [http://cellpublisher.gobics.de/subtinteract/interactionList/2/CopZ CopZ] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://cellpublisher.gobics.de/view/GqbblRJkBH metal ion homeostasis]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://cellpublisher.gobics.de/view/GqbblRJkBH metal ion homeostasis]''' | ||
Revision as of 09:32, 7 August 2011
- Description: copper transport protein, metallochaperone
| Gene name | copZ |
| Synonyms | yvgY |
| Essential | no |
| Product | copper transport protein, metallochaperone |
| Function | resistance to copper |
| Interactions involving this protein in SubtInteract: CopZ | |
| Metabolic function and regulation of this protein in SubtiPathways: metal ion homeostasis | |
| MW, pI | 7 kDa, 4.162 |
| Gene length, protein length | 207 bp, 69 aa |
| Immediate neighbours | copA, csoR |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 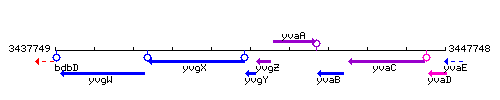
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
transporters/ other, trace metal homeostasis (Cu, Zn, Ni, Mn, Mo), resistance against toxic metals
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33510
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): carries a tetranuclear Cu(I) cluster (as [Cu4(S-Cys)4(N-His)2] cluster) PubMed
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure: 2QIF
- UniProt: O32221
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor: SigA PubMed
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
John Helmann, Cornell University, USA Homepage
Your additional remarks
References
Chloe Singleton, Stephen Hearnshaw, Liang Zhou, Nick E Le Brun, Andrew M Hemmings
Mechanistic insights into Cu(I) cluster transfer between the chaperone CopZ and its cognate Cu(I)-transporting P-type ATPase, CopA.
Biochem J: 2009, 424(3);347-56
[PubMed:19751213]
[WorldCat.org]
[DOI]
(I e)
Stephen Hearnshaw, Claire West, Chloe Singleton, Liang Zhou, Margaret A Kihlken, Richard W Strange, Nick E Le Brun, Andrew M Hemmings
A tetranuclear Cu(I) cluster in the metallochaperone protein CopZ.
Biochemistry: 2009, 48(40);9324-6
[PubMed:19746989]
[WorldCat.org]
[DOI]
(I p)
Agustina Rodriguez-Granillo, Pernilla Wittung-Stafshede
Tuning of copper-loop flexibility in Bacillus subtilis CopZ copper chaperone: role of conserved residues.
J Phys Chem B: 2009, 113(7);1919-32
[PubMed:19170606]
[WorldCat.org]
[DOI]
(P p)
Agustina Rodriguez-Granillo, Pernilla Wittung-Stafshede
Structure and dynamics of Cu(I) binding in copper chaperones Atox1 and CopZ: a computer simulation study.
J Phys Chem B: 2008, 112(15);4583-93
[PubMed:18361527]
[WorldCat.org]
[DOI]
(P p)
Gregory T Smaldone, John D Helmann
CsoR regulates the copper efflux operon copZA in Bacillus subtilis.
Microbiology (Reading): 2007, 153(Pt 12);4123-4128
[PubMed:18048925]
[WorldCat.org]
[DOI]
(P p)
Irina M Solovieva, Karl-Dieter Entian
Metalloregulation in Bacillus subtilis: the copZ chromosomal gene is involved in cadmium resistance.
FEMS Microbiol Lett: 2004, 236(1);115-22
[PubMed:15212800]
[WorldCat.org]
[DOI]
(P p)
Gilles P M Borrelly, Claudia A Blindauer, Ralf Schmid, Clive S Butler, Chris E Cooper, Ian Harvey, Peter J Sadler, Nigel J Robinson
A novel copper site in a cyanobacterial metallochaperone.
Biochem J: 2004, 378(Pt 2);293-7
[PubMed:14711369]
[WorldCat.org]
[DOI]
(I p)
Ahmed Gaballa, Min Cao, John D Helmann
Two MerR homologues that affect copper induction of the Bacillus subtilis copZA operon.
Microbiology (Reading): 2003, 149(Pt 12);3413-3421
[PubMed:14663075]
[WorldCat.org]
[DOI]
(P p)
Lucia Banci, Ivano Bertini, Simone Ciofi-Baffoni, Leonardo Gonnelli, Xun-Cheng Su
Structural basis for the function of the N-terminal domain of the ATPase CopA from Bacillus subtilis.
J Biol Chem: 2003, 278(50);50506-13
[PubMed:14514665]
[WorldCat.org]
[DOI]
(P p)
Ahmed Gaballa, John D Helmann
Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems.
Biometals: 2003, 16(4);497-505
[PubMed:12779235]
[WorldCat.org]
[DOI]
(P p)
David S Radford, Margaret A Kihlken, Gilles P M Borrelly, Colin R Harwood, Nick E Le Brun, Jennifer S Cavet
CopZ from Bacillus subtilis interacts in vivo with a copper exporting CPx-type ATPase CopA.
FEMS Microbiol Lett: 2003, 220(1);105-12
[PubMed:12644235]
[WorldCat.org]
[DOI]
(P p)
Lucia Banci, Ivano Bertini, Simone Ciofi-Baffoni, Rebecca Del Conte, Leonardo Gonnelli
Understanding copper trafficking in bacteria: interaction between the copper transport protein CopZ and the N-terminal domain of the copper ATPase CopA from Bacillus subtilis.
Biochemistry: 2003, 42(7);1939-49
[PubMed:12590580]
[WorldCat.org]
[DOI]
(P p)
Lucia Banci, Ivano Bertini, Simone Ciofi-Baffoni, Mariapina D'Onofrio, Leonardo Gonnelli, Frutos Carlos Marhuenda-Egea, Francisco Javier Ruiz-Dueñas
Solution structure of the N-terminal domain of a potential copper-translocating P-type ATPase from Bacillus subtilis in the apo and Cu(I) loaded states.
J Mol Biol: 2002, 317(3);415-29
[PubMed:11922674]
[WorldCat.org]
[DOI]
(P p)