Difference between revisions of "ClpX"
| Line 18: | Line 18: | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://subtiwiki.uni-goettingen.de/interact/ ''Subt''Interact]''': [http://subtiwiki.uni-goettingen.de/interact/index.php?protein=ClpX ClpX] | |colspan="2" style="background:#FAF8CC;" align="center"| '''Interactions involving this protein in [http://subtiwiki.uni-goettingen.de/interact/ ''Subt''Interact]''': [http://subtiwiki.uni-goettingen.de/interact/index.php?protein=ClpX ClpX] | ||
|- | |- | ||
| − | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/ | + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/subtipathways/search.php?enzyme=clpX clpX]''' |
|- | |- | ||
|style="background:#ABCDEF;" align="center"| '''MW, pI''' || 46 kDa, 4.645 | |style="background:#ABCDEF;" align="center"| '''MW, pI''' || 46 kDa, 4.645 | ||
Revision as of 11:23, 7 January 2014
- Description: ATP-dependent Clp protease ATP-binding subunit (class III heat-shock protein)
| Gene name | clpX |
| Synonyms | |
| Essential | no |
| Product | ATP-dependent Clp protease ATP-binding subunit |
| Function | protein degradation |
| Gene expression levels in SubtiExpress: clpX | |
| Interactions involving this protein in SubtInteract: ClpX | |
| Metabolic function and regulation of this protein in SubtiPathways: clpX | |
| MW, pI | 46 kDa, 4.645 |
| Gene length, protein length | 1260 bp, 420 aa |
| Immediate neighbours | lonB, tig |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 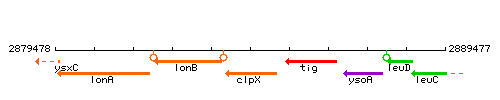
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed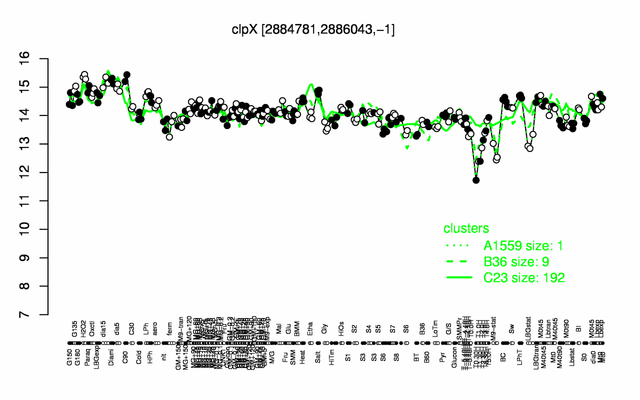
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU28220
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATPase/chaperone
- Protein family: clpX chaperone family (according to Swiss-Prot) ClpX (IP004487) InterPro, AAA+ -type ATPase (IPR013093) InterPro (PF07724) PFAM
Targets of ClpX-ClpP-dependent protein degradation
Extended information on the protein
- Kinetic information:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
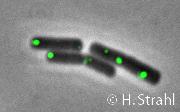
- Localization: cytoplasmic polar clusters, excluded from the nucleoid, induced clustering upon heat shock, colocalization with ClpP PubMed
Database entries
- Structure: homologue structure resolved 1UM8, structural model of B. subtilis ClpX available from hstrahl
- UniProt: P50866
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: clpX PubMed
- Additional information:
- The mRNA has a long 5' leader region. This may indicate RNA-based regulation PubMed
Biological materials
- Mutant: clpX::kan, clpX::spec and clpX::cat available from the Hamoen] Lab
- Expression vector:
- lacZ fusion:
- GFP fusion: C-terminal GFP fusions (both single copy and 2th copy in amyE locus, also as CFP and YFP variants) available from the Hamoen] Lab
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Newcastle University, UK homepage
Your additional remarks
References
Reviews
Additional reviews: PubMed
Aurelia Battesti, Susan Gottesman
Roles of adaptor proteins in regulation of bacterial proteolysis.
Curr Opin Microbiol: 2013, 16(2);140-7
[PubMed:23375660]
[WorldCat.org]
[DOI]
(I p)
David W Adams, Jeff Errington
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.
Nat Rev Microbiol: 2009, 7(9);642-53
[PubMed:19680248]
[WorldCat.org]
[DOI]
(I p)
Dorte Frees, Kirsi Savijoki, Pekka Varmanen, Hanne Ingmer
Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria.
Mol Microbiol: 2007, 63(5);1285-95
[PubMed:17302811]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Additional publications: PubMed