Difference between revisions of "Bpr"
| Line 45: | Line 45: | ||
* '''Locus tag:''' BSU15300 | * '''Locus tag:''' BSU15300 | ||
| + | |||
| + | [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=bpr_1599283_1603584_1 Expression] | ||
===Phenotypes of a mutant === | ===Phenotypes of a mutant === | ||
Revision as of 09:06, 25 January 2012
- Description: bacillopeptidase F
| Gene name | bpr |
| Synonyms | bpf |
| Essential | no |
| Product | bacillopeptidase F |
| Function | protein degradation |
| MW, pI | 154 kDa, 4.976 |
| Gene length, protein length | 4299 bp, 1433 aa |
| Immediate neighbours | ftsZ, spoIIGA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 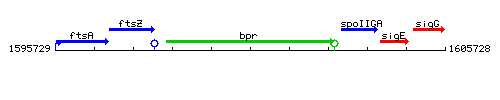
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
utilization of nitrogen sources other than amino acids, proteolysis
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU15300
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: peptidase S8 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: secreted (according to Swiss-Prot), extracellular (signal peptide) PubMed
Database entries
- Structure:
- UniProt: P16397
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- repressed by glucose (7.7-fold) PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications
Birgit Voigt, Haike Antelmann, Dirk Albrecht, Armin Ehrenreich, Karl-Heinz Maurer, Stefan Evers, Gerhard Gottschalk, Jan Maarten van Dijl, Thomas Schweder, Michael Hecker
Cell physiology and protein secretion of Bacillus licheniformis compared to Bacillus subtilis.
J Mol Microbiol Biotechnol: 2009, 16(1-2);53-68
[PubMed:18957862]
[WorldCat.org]
[DOI]
(I p)
Jan-Willem Veening, Oleg A Igoshin, Robyn T Eijlander, Reindert Nijland, Leendert W Hamoen, Oscar P Kuipers
Transient heterogeneity in extracellular protease production by Bacillus subtilis.
Mol Syst Biol: 2008, 4;184
[PubMed:18414485]
[WorldCat.org]
[DOI]
(I p)
Kensuke Tsukahara, Mitsuo Ogura
Characterization of DegU-dependent expression of bpr in Bacillus subtilis.
FEMS Microbiol Lett: 2008, 280(1);8-13
[PubMed:18194340]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
A Sloma, G A Rufo, C F Rudolph, B J Sullivan, K A Theriault, J Pero
Bacillopeptidase F of Bacillus subtilis: purification of the protein and cloning of the gene.
J Bacteriol: 1990, 172(3);1470-7
[PubMed:2106512]
[WorldCat.org]
[DOI]
(P p)