Difference between revisions of "BmrR"
(→References) |
|||
| Line 48: | Line 48: | ||
| + | |||
| + | = Categories containing this gene/protein = | ||
| + | {{SubtiWiki category|[[transcription factors and their control]]}}, | ||
| + | {{SubtiWiki category|[[general stress proteins (controlled by SigB)]]}}, | ||
| + | {{SubtiWiki category|[[resistance against toxins/ antibiotics]]}}, | ||
| + | {{SubtiWiki category|[[membrane proteins]]}} | ||
=The protein= | =The protein= | ||
Revision as of 15:28, 30 November 2010
| Gene name | bmrR |
| Synonyms | |
| Essential | no |
| Product | transcriptional activator |
| Function | regulation of multidrug resistance |
| MW, pI | 32 kDa, 5.187 |
| Gene length, protein length | 834 bp, 278 aa |
| Immediate neighbours | bmr, bkdB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 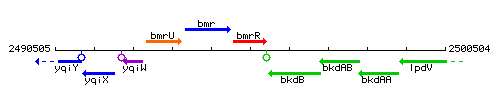
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU24020
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
Categories containing this gene/protein
transcription factors and their control, general stress proteins (controlled by SigB), resistance against toxins/ antibiotics, membrane proteins
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- UniProt: P39075
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Richard Brennan, Houston, Texas, USA Homepage
Your additional remarks
References
Muthiah Kumaraswami, Kate J Newberry, Richard G Brennan
Conformational plasticity of the coiled-coil domain of BmrR is required for bmr operator binding: the structure of unliganded BmrR.
J Mol Biol: 2010, 398(2);264-75
[PubMed:20230832]
[WorldCat.org]
[DOI]
(I p)
A Petersohn, M Brigulla, S Haas, J D Hoheisel, U Völker, M Hecker
Global analysis of the general stress response of Bacillus subtilis.
J Bacteriol: 2001, 183(19);5617-31
[PubMed:11544224]
[WorldCat.org]
[DOI]
(P p)
Anja Petersohn, Haike Antelmann, Ulf Gerth, Michael Hecker
Identification and transcriptional analysis of new members of the sigmaB regulon in Bacillus subtilis.
Microbiology (Reading): 1999, 145 ( Pt 4);869-880
[PubMed:10220166]
[WorldCat.org]
[DOI]
(P p)
N N Baranova, A Danchin, A A Neyfakh
Mta, a global MerR-type regulator of the Bacillus subtilis multidrug-efflux transporters.
Mol Microbiol: 1999, 31(5);1549-59
[PubMed:10200972]
[WorldCat.org]
[DOI]
(P p)
M Ahmed, L Lyass, P N Markham, S S Taylor, N Vázquez-Laslop, A A Neyfakh
Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated.
J Bacteriol: 1995, 177(14);3904-10
[PubMed:7608059]
[WorldCat.org]
[DOI]
(P p)
M Ahmed, C M Borsch, S S Taylor, N Vázquez-Laslop, A A Neyfakh
A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates.
J Biol Chem: 1994, 269(45);28506-13
[PubMed:7961792]
[WorldCat.org]
(P p)