BioB
- Description: biotin synthase
| Gene name | bioB |
| Synonyms | |
| Essential | no |
| Product | biotin synthase) |
| Function | biosynthesis of biotin |
| Gene expression levels in SubtiExpress: bioB | |
| Metabolic function and regulation of this protein in SubtiPathways: bioB | |
| MW, pI | 36 kDa, 5.554 |
| Gene length, protein length | 1005 bp, 335 aa |
| Immediate neighbours | bioI, bioD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 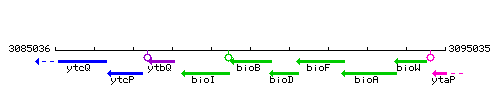
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed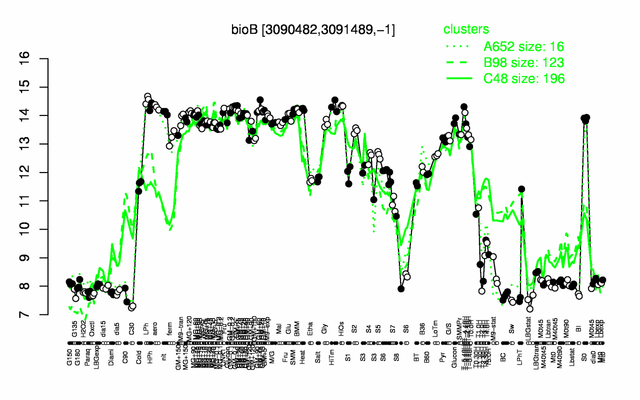
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU30200
Phenotypes of a mutant
Database entries
- BsubCyc: BSU30200
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Dethiobiotin + sulfur + 2 S-adenosyl-L-methionine = biotin + 2 L-methionine + 2 5'-deoxyadenosine (according to Swiss-Prot)
- Protein family: Biotin synthase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): contains an iron-sulfur cluster
- Effectors of protein activity:
Database entries
- BsubCyc: BSU30200
- Structure:
- UniProt: P53557
- KEGG entry: [3]
- E.C. number: 2.8.1.6
Additional information
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 1660 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 4582 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 1227 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1151 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Steven Lin, John E Cronan
Closing in on complete pathways of biotin biosynthesis.
Mol Biosyst: 2011, 7(6);1811-21
[PubMed:21437340]
[WorldCat.org]
[DOI]
(I p)
Original publications
Ulf Gerth, Holger Kock, Ilja Kusters, Stephan Michalik, Robert L Switzer, Michael Hecker
Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis.
J Bacteriol: 2008, 190(1);321-31
[PubMed:17981983]
[WorldCat.org]
[DOI]
(I p)
J B Perkins, S Bower, C L Howitt, R R Yocum, J Pero
Identification and characterization of transcripts from the biotin biosynthetic operon of Bacillus subtilis.
J Bacteriol: 1996, 178(21);6361-5
[PubMed:8892842]
[WorldCat.org]
[DOI]
(P p)
S Bower, J B Perkins, R R Yocum, C L Howitt, P Rahaim, J Pero
Cloning, sequencing, and characterization of the Bacillus subtilis biotin biosynthetic operon.
J Bacteriol: 1996, 178(14);4122-30
[PubMed:8763940]
[WorldCat.org]
[DOI]
(P p)