Difference between revisions of "BdbD"
| Line 129: | Line 129: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 127 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 656 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 09:51, 17 April 2014
- Description: thiol-disulfide oxidoreductase, required for the formation of thiol disulfide bonds in several proteins
| Gene name | bdbD |
| Synonyms | yvgV |
| Essential | no |
| Product | thiol-disulfide oxidoreductase |
| Function | oxidative folding of proteins |
| Gene expression levels in SubtiExpress: bdbD | |
| Metabolic function and regulation of this protein in SubtiPathways: BdbD | |
| MW, pI | 24 kDa, 5.089 |
| Gene length, protein length | 666 bp, 222 aa |
| Immediate neighbours | bdbC, cadA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed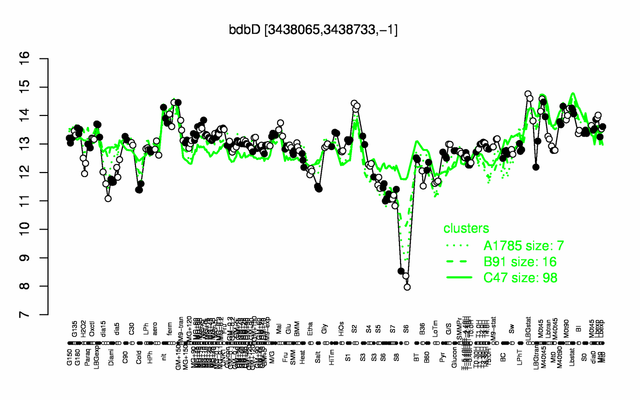
| |
Contents
Categories containing this gene/protein
genetic competence, chaperones/ protein folding, sporulation proteins, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33480
Phenotypes of a mutant
- loss of transformability PubMed
- sensitive to osmotic shock PubMed
- several proteins are absent from the membrane proteome of a bdbC-bdbD mutant: PubMed
Database entries
- BsubCyc: BSU33480
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: DsbA subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s): Ca (2+) PubMed
- Effectors of protein activity:
- Localization: membrane, faced to the outer side of the membrane PubMed
Database entries
- BsubCyc: BSU33480
- UniProt: O32218
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Vivianne J Goosens, Ruben A T Mars, Michiel Akeroyd, Andre Vente, Annette Dreisbach, Emma L Denham, Thijs R H M Kouwen, Tjeerd van Rij, Maurien Olsthoorn, Jan Maarten van Dijl
Is proteomics a reliable tool to probe the oxidative folding of bacterial membrane proteins?
Antioxid Redox Signal: 2013, 18(10);1159-64
[PubMed:22540663]
[WorldCat.org]
[DOI]
(I p)
Allister Crow, Allison Lewin, Oliver Hecht, Mirja Carlsson Möller, Geoffrey R Moore, Lars Hederstedt, Nick E Le Brun
Crystal structure and biophysical properties of Bacillus subtilis BdbD. An oxidizing thiol:disulfide oxidoreductase containing a novel metal site.
J Biol Chem: 2009, 284(35);23719-33
[PubMed:19535335]
[WorldCat.org]
[DOI]
(P p)
Inês Chen, Roberta Provvedi, David Dubnau
A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis.
J Biol Chem: 2006, 281(31);21720-21727
[PubMed:16751195]
[WorldCat.org]
[DOI]
(P p)
Irena Draskovic, David Dubnau
Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds.
Mol Microbiol: 2005, 55(3);881-96
[PubMed:15661011]
[WorldCat.org]
[DOI]
(P p)
Ritsuko Kuwana, Yasuhiro Kasahara, Machiko Fujibayashi, Hiromu Takamatsu, Naotake Ogasawara, Kazuhito Watabe
Proteomics characterization of novel spore proteins of Bacillus subtilis.
Microbiology (Reading): 2002, 148(Pt 12);3971-3982
[PubMed:12480901]
[WorldCat.org]
[DOI]
(P p)
Ronald Dorenbos, Torsten Stein, Jorrit Kabel, Claude Bruand, Albert Bolhuis, Sierd Bron, Wim J Quax, Jan Maarten Van Dijl
Thiol-disulfide oxidoreductases are essential for the production of the lantibiotic sublancin 168.
J Biol Chem: 2002, 277(19);16682-8
[PubMed:11872755]
[WorldCat.org]
[DOI]
(P p)
Lýdur S Erlendsson, Lars Hederstedt
Mutations in the thiol-disulfide oxidoreductases BdbC and BdbD can suppress cytochrome c deficiency of CcdA-defective Bacillus subtilis cells.
J Bacteriol: 2002, 184(5);1423-9
[PubMed:11844773]
[WorldCat.org]
[DOI]
(P p)
Rob Meima, Caroline Eschevins, Sabine Fillinger, Albert Bolhuis, Leendert W Hamoen, Ronald Dorenbos, Wim J Quax, Jan Maarten van Dijl, Roberta Provvedi, Ines Chen, David Dubnau, Sierd Bron
The bdbDC operon of Bacillus subtilis encodes thiol-disulfide oxidoreductases required for competence development.
J Biol Chem: 2002, 277(9);6994-7001
[PubMed:11744713]
[WorldCat.org]
[DOI]
(P p)