Difference between revisions of "AtpC"
| Line 122: | Line 122: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 1924 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 4205 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 09:36, 17 April 2014
- Description: ATP synthase, part of the F1 complex (subunit epsilon)
| Gene name | atpC |
| Synonyms | |
| Essential | no |
| Product | ATP synthase (subunit epsilon)) |
| Function | ATP synthesis |
| Gene expression levels in SubtiExpress: atpC | |
| Interactions involving this protein in SubtInteract: AtpC | |
| MW, pI | 14 kDa, 5.262 |
| Gene length, protein length | 396 bp, 132 aa |
| Immediate neighbours | ywmA, atpD |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed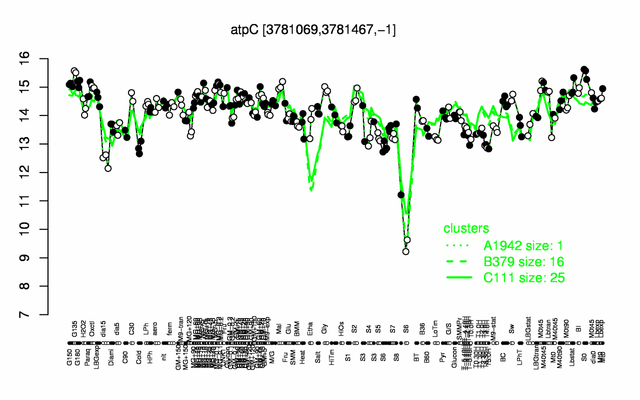
| |
Contents
Categories containing this gene/protein
ATP synthesis, membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU36800
Phenotypes of a mutant
Database entries
- BsubCyc: BSU36800
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP synthesis see a video
- Protein family: ATPase epsilon chain family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- cell membrane (Heterogeneous) PubMed
- peripheral via theF0 complex
Database entries
- BsubCyc: BSU36800
- Structure: see here an overview on ATPase structure
- UniProt: P37812
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
John E Walker
The ATP synthase: the understood, the uncertain and the unknown.
Biochem Soc Trans: 2013, 41(1);1-16
[PubMed:23356252]
[WorldCat.org]
[DOI]
(I p)
Ryota Iino, Hiroyuki Noji
Operation mechanism of F(o) F(1)-adenosine triphosphate synthase revealed by its structure and dynamics.
IUBMB Life: 2013, 65(3);238-46
[PubMed:23341301]
[WorldCat.org]
[DOI]
(I p)
Hendrik Sielaff, Michael Börsch
Twisting and subunit rotation in single F(O)(F1)-ATP synthase.
Philos Trans R Soc Lond B Biol Sci: 2013, 368(1611);20120024
[PubMed:23267178]
[WorldCat.org]
[DOI]
(I e)
Alan E Senior
Two ATPases.
J Biol Chem: 2012, 287(36);30049-62
[PubMed:22822068]
[WorldCat.org]
[DOI]
(I p)
Daichi Okuno, Ryota Iino, Hiroyuki Noji
Rotation and structure of FoF1-ATP synthase.
J Biochem: 2011, 149(6);655-64
[PubMed:21524994]
[WorldCat.org]
[DOI]
(I p)
Christoph von Ballmoos, Alexander Wiedenmann, Peter Dimroth
Essentials for ATP synthesis by F1F0 ATP synthases.
Annu Rev Biochem: 2009, 78;649-72
[PubMed:19489730]
[WorldCat.org]
[DOI]
(I p)
Joachim Weber
ATP synthase--the structure of the stator stalk.
Trends Biochem Sci: 2007, 32(2);53-6
[PubMed:17208001]
[WorldCat.org]
[DOI]
(P p)
Joachim Weber
ATP synthase: subunit-subunit interactions in the stator stalk.
Biochim Biophys Acta: 2006, 1757(9-10);1162-70
[PubMed:16730323]
[WorldCat.org]
[DOI]
(P p)
Original publications
Junya Mizumoto, Yuka Kikuchi, Yo-Hei Nakanishi, Naoto Mouri, Anrong Cai, Tokushiro Ohta, Takamitsu Haruyama, Yasuyuki Kato-Yamada
ε subunit of Bacillus subtilis F1-ATPase relieves MgADP inhibition.
PLoS One: 2013, 8(8);e73888
[PubMed:23967352]
[WorldCat.org]
[DOI]
(I e)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
M Santana, M S Ionescu, A Vertes, R Longin, F Kunst, A Danchin, P Glaser
Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants.
J Bacteriol: 1994, 176(22);6802-11
[PubMed:7961438]
[WorldCat.org]
[DOI]
(P p)