Difference between revisions of "Asd"
| Line 129: | Line 129: | ||
** number of protein molecules per cell (minimal medium with glucose and ammonium): 5224 {{PubMed|24696501}} | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 5224 {{PubMed|24696501}} | ||
** number of protein molecules per cell (complex medium with amino acids, without glucose): 8453 {{PubMed|24696501}} | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 8453 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 932 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 3982 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 13:32, 17 April 2014
- Description: aspartate-semialdehyde dehydrogenase
| Gene name | asd |
| Synonyms | |
| Essential | yes PubMed |
| Product | aspartate-semialdehyde dehydrogenase |
| Function | biosynthesis of threonine, lysine, dipicolic acid, peptidoglycan |
| Gene expression levels in SubtiExpress: asd | |
| Interactions involving this protein in SubtInteract: Asd | |
| Metabolic function and regulation of this protein in SubtiPathways: asd | |
| MW, pI | 37 kDa, 4.971 |
| Gene length, protein length | 1038 bp, 346 aa |
| Immediate neighbours | spoVFB, dapG |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed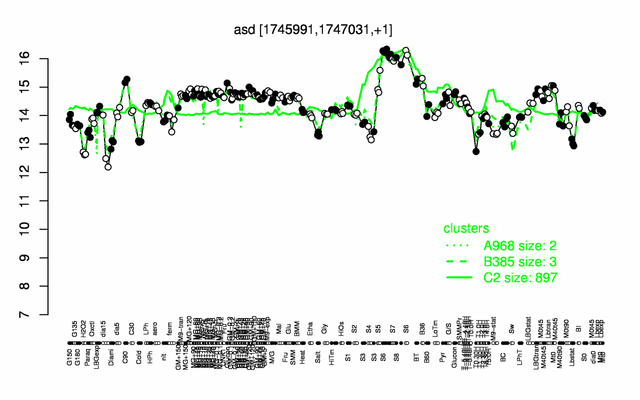
| |
Contents
Categories containing this gene/protein
cell wall synthesis, biosynthesis/ acquisition of amino acids, Biosynthesis of cell wall components, sporulation proteins, essential genes, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU16750
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU16750
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-aspartate 4-semialdehyde + phosphate + NADP+ = L-4-aspartyl phosphate + NADPH (according to Swiss-Prot)
- Protein family: aspartate-semialdehyde dehydrogenase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification: phosphorylation on Ser-98 AND Tyr-146 PubMed
- Effectors of protein activity:
Database entries
- BsubCyc: BSU16750
- UniProt: Q04797
- KEGG entry: [3]
- E.C. number: 1.2.1.11
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 5224 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 8453 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 932 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 3982 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Leif Steil, Mónica Serrano, Adriano O Henriques, Uwe Völker
Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis.
Microbiology (Reading): 2005, 151(Pt 2);399-420
[PubMed:15699190]
[WorldCat.org]
[DOI]
(P p)
R A Daniel, J Errington
Cloning, DNA sequence, functional analysis and transcriptional regulation of the genes encoding dipicolinic acid synthetase required for sporulation in Bacillus subtilis.
J Mol Biol: 1993, 232(2);468-83
[PubMed:8345520]
[WorldCat.org]
[DOI]
(P p)
N Y Chen, S Q Jiang, D A Klein, H Paulus
Organization and nucleotide sequence of the Bacillus subtilis diaminopimelate operon, a cluster of genes encoding the first three enzymes of diaminopimelate synthesis and dipicolinate synthase.
J Biol Chem: 1993, 268(13);9448-65
[PubMed:8098035]
[WorldCat.org]
(P p)