AroH
- Description: chorismate mutase (isozymes 1 and 2)
| Gene name | aroH |
| Synonyms | |
| Essential | no |
| Product | chorismate mutase (isozymes 1 and 2) |
| Function | biosynthesis of aromatic amino acids |
| Gene expression levels in SubtiExpress: aroH | |
| Metabolic function and regulation of this protein in SubtiPathways: aroH | |
| MW, pI | 14 kDa, 5.524 |
| Gene length, protein length | 381 bp, 127 aa |
| Immediate neighbours | trpE, aroB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 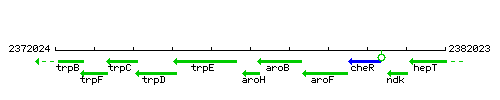
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed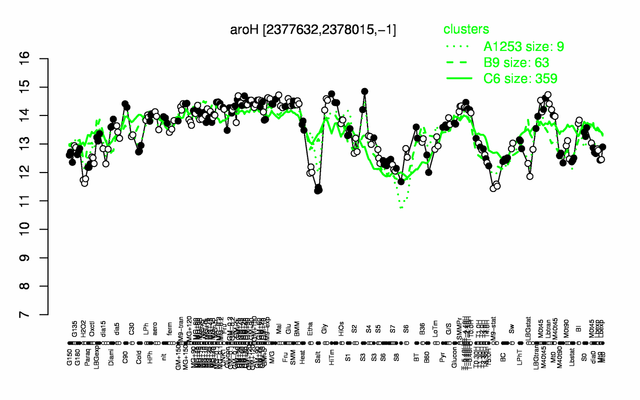
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU22690
Phenotypes of a mutant
Database entries
- BsubCyc: BSU22690
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Chorismate = prephenate (according to Swiss-Prot)
- Protein family: BCKDHA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU22690
- UniProt: P19080
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1441 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 1006 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 804 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Daniel Burschowsky, André van Eerde, Mats Ökvist, Alexander Kienhöfer, Peter Kast, Donald Hilvert, Ute Krengel
Electrostatic transition state stabilization rather than reactant destabilization provides the chemical basis for efficient chorismate mutase catalysis.
Proc Natl Acad Sci U S A: 2014, 111(49);17516-21
[PubMed:25422475]
[WorldCat.org]
[DOI]
(I p)
Alexander Kienhöfer, Peter Kast, Donald Hilvert
Selective stabilization of the chorismate mutase transition state by a positively charged hydrogen bond donor.
J Am Chem Soc: 2003, 125(11);3206-7
[PubMed:12630863]
[WorldCat.org]
[DOI]
(P p)
P Kast, C Grisostomi, I A Chen, S Li, U Krengel, Y Xue, D Hilvert
A strategically positioned cation is crucial for efficient catalysis by chorismate mutase.
J Biol Chem: 2000, 275(47);36832-8
[PubMed:10960481]
[WorldCat.org]
[DOI]
(P p)
P Kast, M Asif-Ullah, N Jiang, D Hilvert
Exploring the active site of chorismate mutase by combinatorial mutagenesis and selection: the importance of electrostatic catalysis.
Proc Natl Acad Sci U S A: 1996, 93(10);5043-8
[PubMed:8643526]
[WorldCat.org]
[DOI]
(P p)
Y M Chook, H Ke, W N Lipscomb
Crystal structures of the monofunctional chorismate mutase from Bacillus subtilis and its complex with a transition state analog.
Proc Natl Acad Sci U S A: 1993, 90(18);8600-3
[PubMed:8378335]
[WorldCat.org]
[DOI]
(P p)
J V Gray, B Golinelli-Pimpaneau, J R Knowles
Monofunctional chorismate mutase from Bacillus subtilis: purification of the protein, molecular cloning of the gene, and overexpression of the gene product in Escherichia coli.
Biochemistry: 1990, 29(2);376-83
[PubMed:2105742]
[WorldCat.org]
[DOI]
(P p)