Difference between revisions of "ArgD"
m (Reverted edits by 134.76.70.252 (talk) to last revision by 134.76.38.147) |
|||
| Line 125: | Line 125: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1476 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 850 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1501 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 13:53, 17 April 2014
- Description: acetylornithine transaminase
| Gene name | argD |
| Synonyms | |
| Essential | no |
| Product | acetylornithine transaminase |
| Function | biosynthesis of arginine |
| Gene expression levels in SubtiExpress: argD | |
| Metabolic function and regulation of this protein in SubtiPathways: argD | |
| MW, pI | 40 kDa, 5.929 |
| Gene length, protein length | 1155 bp, 385 aa |
| Immediate neighbours | argB, carA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed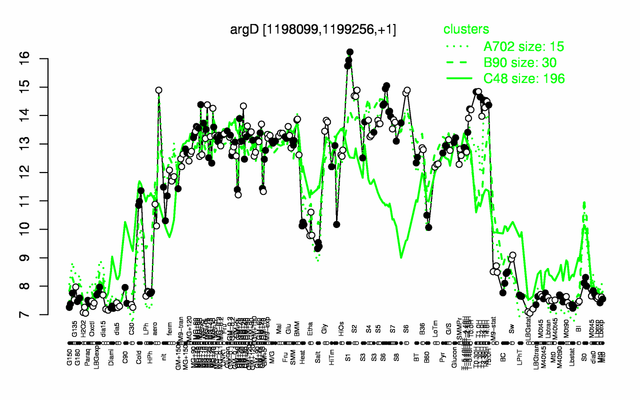
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU11220
Phenotypes of a mutant
Database entries
- BsubCyc: BSU11220
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: N(2)-acetyl-L-ornithine + 2-oxoglutarate = N-acetyl-L-glutamate 5-semialdehyde + L-glutamate (according to Swiss-Prot)
- Protein family: ArgD subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU11220
- Structure:
- UniProt: P36839
- KEGG entry: [3]
- E.C. number: 2.6.1.11
Additional information
Expression and regulation
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 1476 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 850 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 1501 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)
A Mountain, N H Mann, R N Munton, S Baumberg
Cloning of a Bacillus subtilis restriction fragment complementing auxotrophic mutants of eight Escherichia coli genes of arginine biosynthesis.
Mol Gen Genet: 1984, 197(1);82-9
[PubMed:6096675]
[WorldCat.org]
[DOI]
(P p)