Difference between revisions of "ArgB"
| Line 119: | Line 119: | ||
=References= | =References= | ||
| − | <pubmed>12107147, </pubmed> | + | <pubmed>6096675,,12107147, </pubmed> |
# Mäder et al. (2002) Transcriptome and Proteome Analysis of ''Bacillus subtilis'' Gene Expression Modulated by Amino Acid Availability. ''J. Bacteriol'' '''184:''' 1844288-4295 [http://www.ncbi.nlm.nih.gov/pubmed/12107147 PubMed] | # Mäder et al. (2002) Transcriptome and Proteome Analysis of ''Bacillus subtilis'' Gene Expression Modulated by Amino Acid Availability. ''J. Bacteriol'' '''184:''' 1844288-4295 [http://www.ncbi.nlm.nih.gov/pubmed/12107147 PubMed] | ||
# Gerth et al. (2008) Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. J Bacteriol 190:321-331 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17981983 PubMed] | # Gerth et al. (2008) Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. J Bacteriol 190:321-331 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17981983 PubMed] | ||
# Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | # Author1, Author2 & Author3 (year) Title ''Journal'' '''volume:''' page-page. [http://www.ncbi.nlm.nih.gov/sites/entrez/PMID PubMed] | ||
Revision as of 16:46, 13 June 2009
- Description: N-acetylglutamate 5-phosphotransferase
| Gene name | argB |
| Synonyms | |
| Essential | no |
| Product | N-acetylglutamate 5-phosphotransferase |
| Function | biosynthesis of arginine |
| MW, pI | 27 kDa, 6.214 |
| Gene length, protein length | 774 bp, 258 aa |
| Immediate neighbours | argJ, argD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 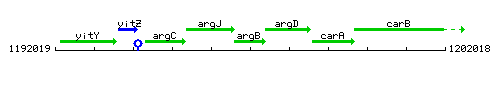
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU11210
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + N-acetyl-L-glutamate = ADP + N-acetyl-L-glutamate 5-phosphate (according to Swiss-Prot)
- Protein family: acetylglutamate kinase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- Structure:
- Swiss prot entry: P68729
- KEGG entry: [3]
- E.C. number: 2.7.2.8
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Regulatory mechanism: AhrC: transcription repression
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Ulrike Mäder, Georg Homuth, Christian Scharf, Knut Büttner, Rüdiger Bode, Michael Hecker
Transcriptome and proteome analysis of Bacillus subtilis gene expression modulated by amino acid availability.
J Bacteriol: 2002, 184(15);4288-95
[PubMed:12107147]
[WorldCat.org]
[DOI]
(P p)
A Mountain, N H Mann, R N Munton, S Baumberg
Cloning of a Bacillus subtilis restriction fragment complementing auxotrophic mutants of eight Escherichia coli genes of arginine biosynthesis.
Mol Gen Genet: 1984, 197(1);82-9
[PubMed:6096675]
[WorldCat.org]
[DOI]
(P p)
- Mäder et al. (2002) Transcriptome and Proteome Analysis of Bacillus subtilis Gene Expression Modulated by Amino Acid Availability. J. Bacteriol 184: 1844288-4295 PubMed
- Gerth et al. (2008) Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. J Bacteriol 190:321-331 PubMed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed