Difference between revisions of "AraA"
(→Expression and regulation) |
(→Database entries) |
||
| Line 78: | Line 78: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=2ajt 2AJT] (from ''Escherichia coli'', 51% identity, 71% similarity) {{PubMed|16756997}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P94523 P94523] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P94523 P94523] | ||
Revision as of 14:06, 17 February 2010
- Description: L-arabinose isomerase
| Gene name | araA |
| Synonyms | |
| Essential | no |
| Product | L-arabinose isomerase |
| Function | arabinose utilization |
| Metabolic function and regulation of this protein in SubtiPathways: Sugar catabolism | |
| MW, pI | 56 kDa, 5.474 |
| Gene length, protein length | 1494 bp, 498 aa |
| Immediate neighbours | araB, abnA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 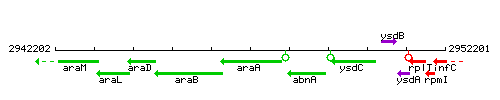
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28800
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: L-arabinose = L-ribulose (according to Swiss-Prot)
- Protein family: arabinose isomerase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: k (cat) of 14,504 min(-1) and k (cat)/K (m) of 121 min(-1) mM(-1) for L-arabinose PubMed
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- UniProt: P94523
- KEGG entry: [3]
- E.C. number: 5.3.1.4
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Jin-Ha Kim, Ponnandy Prabhu, Marimuthu Jeya, Manish Kumar Tiwari, Hee-Jung Moon, Raushan Kumar Singh, Jung-Kul Lee
Characterization of an L-arabinose isomerase from Bacillus subtilis.
Appl Microbiol Biotechnol: 2010, 85(6);1839-47
[PubMed:19727704]
[WorldCat.org]
[DOI]
(I p)
Irina Saraiva Franco, Luís Jaime Mota, Cláudio Manuel Soares, Isabel de Sá-Nogueira
Functional domains of the Bacillus subtilis transcription factor AraR and identification of amino acids important for nucleoprotein complex assembly and effector binding.
J Bacteriol: 2006, 188(8);3024-36
[PubMed:16585763]
[WorldCat.org]
[DOI]
(P p)
José Manuel Inácio, Carla Costa, Isabel de Sá-Nogueira
Distinct molecular mechanisms involved in carbon catabolite repression of the arabinose regulon in Bacillus subtilis.
Microbiology (Reading): 2003, 149(Pt 9);2345-2355
[PubMed:12949161]
[WorldCat.org]
[DOI]
(P p)
L J Mota, L M Sarmento, I de Sá-Nogueira
Control of the arabinose regulon in Bacillus subtilis by AraR in vivo: crucial roles of operators, cooperativity, and DNA looping.
J Bacteriol: 2001, 183(14);4190-201
[PubMed:11418559]
[WorldCat.org]
[DOI]
(P p)
L J Mota, P Tavares, I Sá-Nogueira
Mode of action of AraR, the key regulator of L-arabinose metabolism in Bacillus subtilis.
Mol Microbiol: 1999, 33(3);476-89
[PubMed:10417639]
[WorldCat.org]
[DOI]
(P p)
Isabel S-Nogueira, Teresa V Nogueira, Snia Soares, Hermnia de Lencastre
The Bacillus subtilis L-arabinose (ara) operon: nucleotide sequence, genetic organization and expression.
Microbiology (Reading): 1997, 143 ( Pt 3);957-969
[PubMed:9084180]
[WorldCat.org]
[DOI]
(P p)
I Sá-Nogueira, H de Lencastre
Cloning and characterization of araA, araB, and araD, the structural genes for L-arabinose utilization in Bacillus subtilis.
J Bacteriol: 1989, 171(7);4088-91
[PubMed:2500424]
[WorldCat.org]
[DOI]
(P p)