Difference between revisions of "AhpF"
(→Expression and regulation) |
(→Database entries) |
||
| Line 78: | Line 78: | ||
=== Database entries === | === Database entries === | ||
| − | * '''Structure:''' | + | * '''Structure:''' [http://www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1HYU 1HYU] (from ''Salmonella typhimurium'', 52% identity, 69% similarity) {{PubMed|11300769}} |
* '''UniProt:''' [http://www.uniprot.org/uniprot/P42974 P42974] | * '''UniProt:''' [http://www.uniprot.org/uniprot/P42974 P42974] | ||
Revision as of 09:32, 18 February 2010
- Description: alkyl hydroperoxide reductase (large subunit) / NADH dehydrogenase
| Gene name | ahpF |
| Synonyms | ndh |
| Essential | no |
| Product | alkyl hydroperoxide reductase (large subunit) / NADH dehydrogenase |
| Function | resistance against peroxide stres |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 54 kDa, 4.705 |
| Gene length, protein length | 1527 bp, 509 aa |
| Immediate neighbours | ahpC, bglA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 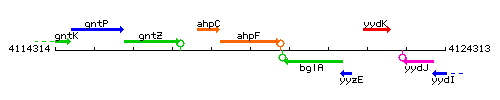
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU40100
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: NADH + acceptor = NAD+ + reduced acceptor (according to Swiss-Prot)
- Protein family: class-II pyridine nucleotide-disulfide oxidoreductase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on (Ser-48 OR Ser-49) PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- UniProt: P42974
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
A Sakai, K Katayama, T Katsuragi, Y Tani
Glycolaldehyde-forming route in Bacillus subtilis in relation to vitamin B6 biosynthesis.
J Biosci Bioeng: 2001, 91(2);147-52
[PubMed:16232966]
[WorldCat.org]
[DOI]
(P p)
A F Herbig, J D Helmann
Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA.
Mol Microbiol: 2001, 41(4);849-59
[PubMed:11532148]
[WorldCat.org]
[DOI]
(P p)
N Bsat, L Chen, J D Helmann
Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes.
J Bacteriol: 1996, 178(22);6579-86
[PubMed:8932315]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, S Engelmann, R Schmid, M Hecker
General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon.
J Bacteriol: 1996, 178(22);6571-8
[PubMed:8932314]
[WorldCat.org]
[DOI]
(P p)