SrfAA
- Description: surfactin synthetase / competence
| Gene name | srfAA |
| Synonyms | comL |
| Essential | no |
| Product | surfactin synthetase / competence |
| Function | antibiotic synthesis |
| Gene expression levels in SubtiExpress: srfAA | |
| MW, pI | 401 kDa, 4.871 |
| Gene length, protein length | 10764 bp, 3588 aa |
| Immediate neighbours | hxlR, srfAB |
| Sequences | Protein DNA Advanced_DNA |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed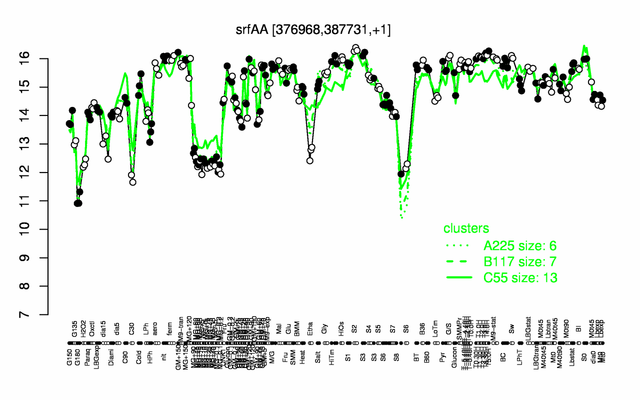
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biosynthesis of antibacterial compounds, membrane proteins, phosphoproteins
This gene is a member of the following regulons
Abh regulon, CodY regulon, ComA regulon, PerR regulon, Spx regulon
The gene
Basic information
- Locus tag: BSU03480
Phenotypes of a mutant
- altered cell death pattern in colonies PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
- A mutation was found in this gene after evolution under relaxed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: ATP-dependent AMP-binding enzyme family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Ser-1006 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane PubMed
Database entries
- Structure:
- UniProt: P27206
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications
Additional publications: PubMed
Munehiro Asally, Mark Kittisopikul, Pau Rué, Yingjie Du, Zhenxing Hu, Tolga Çağatay, Andra B Robinson, Hongbing Lu, Jordi Garcia-Ojalvo, Gürol M Süel
Localized cell death focuses mechanical forces during 3D patterning in a biofilm.
Proc Natl Acad Sci U S A: 2012, 109(46);18891-6
[PubMed:23012477]
[WorldCat.org]
[DOI]
(I p)
Christopher T Brown, Laura K Fishwick, Binna M Chokshi, Marissa A Cuff, Jay M Jackson, Travis Oglesby, Alison T Rioux, Enrique Rodriguez, Gregory S Stupp, Austin H Trupp, James S Woollcombe-Clarke, Tracy N Wright, William J Zaragoza, Jennifer C Drew, Eric W Triplett, Wayne L Nicholson
Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation.
Appl Environ Microbiol: 2011, 77(19);6867-77
[PubMed:21821766]
[WorldCat.org]
[DOI]
(I p)
Femke I Kraas, Verena Helmetag, Melanie Wittmann, Matthias Strieker, Mohamed A Marahiel
Functional dissection of surfactin synthetase initiation module reveals insights into the mechanism of lipoinitiation.
Chem Biol: 2010, 17(8);872-80
[PubMed:20797616]
[WorldCat.org]
[DOI]
(I p)
Irnov Irnov, Cynthia M Sharma, Jörg Vogel, Wade C Winkler
Identification of regulatory RNAs in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(19);6637-51
[PubMed:20525796]
[WorldCat.org]
[DOI]
(I p)
F Coutte, V Leclère, M Béchet, J-S Guez, D Lecouturier, M Chollet-Imbert, P Dhulster, P Jacques
Effect of pps disruption and constitutive expression of srfA on surfactin productivity, spreading and antagonistic properties of Bacillus subtilis 168 derivatives.
J Appl Microbiol: 2010, 109(2);480-491
[PubMed:20148996]
[WorldCat.org]
[DOI]
(I p)
Thomas E Angelini, Marcus Roper, Roberto Kolter, David A Weitz, Michael P Brenner
Bacillus subtilis spreads by surfing on waves of surfactant.
Proc Natl Acad Sci U S A: 2009, 106(43);18109-13
[PubMed:19826092]
[WorldCat.org]
[DOI]
(I p)
Daniel López, Hera Vlamakis, Richard Losick, Roberto Kolter
Paracrine signaling in a bacterium.
Genes Dev: 2009, 23(14);1631-8
[PubMed:19605685]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Mitsuo Ogura, Yasutaro Fujita
Bacillus subtilis rapD, a direct target of transcription repression by RghR, negatively regulates srfA expression.
FEMS Microbiol Lett: 2007, 268(1);73-80
[PubMed:17227471]
[WorldCat.org]
[DOI]
(P p)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Paul D Straight, Michael A Fischbach, Christopher T Walsh, David Z Rudner, Roberto Kolter
A singular enzymatic megacomplex from Bacillus subtilis.
Proc Natl Acad Sci U S A: 2007, 104(1);305-10
[PubMed:17190806]
[WorldCat.org]
[DOI]
(P p)
Kentaro Hayashi, Taku Ohsawa, Kazuo Kobayashi, Naotake Ogasawara, Mitsuo Ogura
The H2O2 stress-responsive regulator PerR positively regulates srfA expression in Bacillus subtilis.
J Bacteriol: 2005, 187(19);6659-67
[PubMed:16166527]
[WorldCat.org]
[DOI]
(P p)
Natalia Comella, Alan D Grossman
Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis.
Mol Microbiol: 2005, 57(4);1159-74
[PubMed:16091051]
[WorldCat.org]
[DOI]
(P p)
Shunji Nakano, Michiko M Nakano, Ying Zhang, Montira Leelakriangsak, Peter Zuber
A regulatory protein that interferes with activator-stimulated transcription in bacteria.
Proc Natl Acad Sci U S A: 2003, 100(7);4233-8
[PubMed:12642660]
[WorldCat.org]
[DOI]
(P p)
P Serror, A L Sonenshein
CodY is required for nutritional repression of Bacillus subtilis genetic competence.
J Bacteriol: 1996, 178(20);5910-5
[PubMed:8830686]
[WorldCat.org]
[DOI]
(P p)
A Luttinger, J Hahn, D Dubnau
Polynucleotide phosphorylase is necessary for competence development in Bacillus subtilis.
Mol Microbiol: 1996, 19(2);343-56
[PubMed:8825779]
[WorldCat.org]
[DOI]
(P p)
D Vollenbroich, N Mehta, P Zuber, J Vater, R M Kamp
Analysis of surfactin synthetase subunits in srfA mutants of Bacillus subtilis OKB105.
J Bacteriol: 1994, 176(2);395-400
[PubMed:8288534]
[WorldCat.org]
[DOI]
(P p)
M Roggiani, D Dubnau
ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA.
J Bacteriol: 1993, 175(10);3182-7
[PubMed:8387999]
[WorldCat.org]
[DOI]
(P p)
J Hahn, D Dubnau
Growth stage signal transduction and the requirements for srfA induction in development of competence.
J Bacteriol: 1991, 173(22);7275-82
[PubMed:1938922]
[WorldCat.org]
[DOI]
(P p)
M M Nakano, L A Xia, P Zuber
Transcription initiation region of the srfA operon, which is controlled by the comP-comA signal transduction system in Bacillus subtilis.
J Bacteriol: 1991, 173(17);5487-93
[PubMed:1715856]
[WorldCat.org]
[DOI]
(P p)