NprE
- Description: extracellular neutral protease B
| Gene name | nprE |
| Synonyms | |
| Essential | no |
| Product | extracellular neutral protease B |
| Function | degradation of proteins |
| MW, pI | 56 kDa, 7.481 |
| Gene length, protein length | 1563 bp, 521 aa |
| Immediate neighbours | yktD, ylaA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 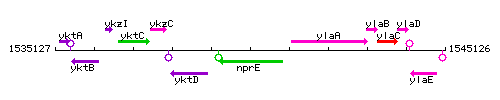
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU14700
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Similar, but not identical, to that of thermolysin (according to Swiss-Prot)
- Protein family: peptidase M4 family (according to Swiss-Prot)
- Paralogous protein(s): NprB
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: secreted (according to Swiss-Prot), extracellular (signal peptide) PubMed
Database entries
- Structure: 1NPC (of Bacillus cereus)
- UniProt: P68736
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: nprE (according to DBTBS)
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Massimiliano Marvasi, Pieter T Visscher, Lilliam Casillas Martinez
Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis.
FEMS Microbiol Lett: 2010, 313(1);1-9
[PubMed:20735481]
[WorldCat.org]
[DOI]
(I p)
Original publications