RpoC
- Description: RNA polymerase beta' subunit
| Gene name | rpoC |
| Synonyms | |
| Essential | yes PubMed |
| Product | RNA polymerase beta' subunit |
| Function | transcription |
| Gene expression levels in SubtiExpress: rpoC | |
| Interactions involving this protein in SubtInteract: RpoC | |
| MW, pI | 133 kDa, 8.863 |
| Gene length, protein length | 3597 bp, 1199 aa |
| Immediate neighbours | rpoB, ybxF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed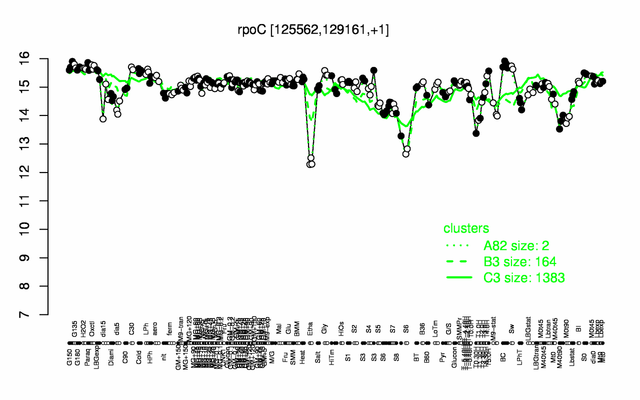
| |
Contents
Categories containing this gene/protein
transcription, essential genes, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU01080
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1) (according to Swiss-Prot)
- Protein family: RNA polymerase beta' chain family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- phosphorylated on Arg-335 and Arg-803 PubMed
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- RpoA-RpoB-RpoC PubMed
- SigA-(RpoB-RpoC) PubMed, SigB-(RpoB-RpoC)
- SigD-(RpoB-RpoC), SigE-(RpoB-RpoC)
- SigF-(RpoB-RpoC), SigG-(RpoB-RpoC)
- SigH-(RpoB-RpoC), SigI-(RpoB-RpoC)
- SigK-(RpoB-RpoC), SigL-(RpoB-RpoC)
- SigM-(RpoB-RpoC), SigV-(RpoB-RpoC)
- SigW-(RpoB-RpoC), SigX-(RpoB-RpoC)
- SigY-(RpoB-RpoC), SigZ-(RpoB-RpoC)
- Xpf-(RpoB-RpoC), YlaC-(RpoB-RpoC)
- YvrHa-RpoC PubMed
- Localization: membrane associated PubMed
Database entries
- Structure:
- UniProt: P37871
- KEGG entry: [2]
- E.C. number: 2.7.7.6
Additional information
Expression and regulation
- Operon:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Lakshminarayan M Iyer, L Aravind
Insights from the architecture of the bacterial transcription apparatus.
J Struct Biol: 2012, 179(3);299-319
[PubMed:22210308]
[WorldCat.org]
[DOI]
(I p)
Original publications
Additional publications: PubMed
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
Elecia B Johnston, Peter J Lewis, Renate Griffith
The interaction of Bacillus subtilis sigmaA with RNA polymerase.
Protein Sci: 2009, 18(11);2287-97
[PubMed:19735077]
[WorldCat.org]
[DOI]
(I p)
Xiao Yang, Seeseei Molimau, Geoff P Doherty, Elecia B Johnston, Jon Marles-Wright, Rosalba Rothnagel, Ben Hankamer, Richard J Lewis, Peter J Lewis
The structure of bacterial RNA polymerase in complex with the essential transcription elongation factor NusA.
EMBO Rep: 2009, 10(9);997-1002
[PubMed:19680289]
[WorldCat.org]
[DOI]
(I p)
Hannes Hahne, Susanne Wolff, Michael Hecker, Dörte Becher
From complementarity to comprehensiveness--targeting the membrane proteome of growing Bacillus subtilis by divergent approaches.
Proteomics: 2008, 8(19);4123-36
[PubMed:18763711]
[WorldCat.org]
[DOI]
(I p)
Claudia Rollenhagen, Haike Antelmann, Janine Kirstein, Olivier Delumeau, Michael Hecker, Michael D Yudkin
Binding of sigma(A) and sigma(B) to core RNA polymerase after environmental stress in Bacillus subtilis.
J Bacteriol: 2003, 185(1);35-40
[PubMed:12486038]
[WorldCat.org]
[DOI]
(P p)
P J Lewis, S D Thaker, J Errington
Compartmentalization of transcription and translation in Bacillus subtilis.
EMBO J: 2000, 19(4);710-8
[PubMed:10675340]
[WorldCat.org]
[DOI]
(P p)
X Yang, C W Price
Streptolydigin resistance can be conferred by alterations to either the beta or beta' subunits of Bacillus subtilis RNA polymerase.
J Biol Chem: 1995, 270(41);23930-3
[PubMed:7592585]
[WorldCat.org]
[DOI]
(P p)
K J Boor, M L Duncan, C W Price
Genetic and transcriptional organization of the region encoding the beta subunit of Bacillus subtilis RNA polymerase.
J Biol Chem: 1995, 270(35);20329-36
[PubMed:7657605]
[WorldCat.org]
[DOI]
(P p)