YhdA
- Description: similar to NADPH-dependent azobenzene FMN reductase
| Gene name | yhdA |
| Synonyms | |
| Essential | no |
| Product | unknown |
| Function | unknown |
| Gene expression levels in SubtiExpress: yhdA | |
| MW, pI | 18 kDa, 6.508 |
| Gene length, protein length | 522 bp, 174 aa |
| Immediate neighbours | yhcZ, yhdB |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 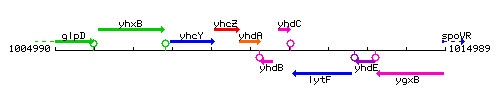
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed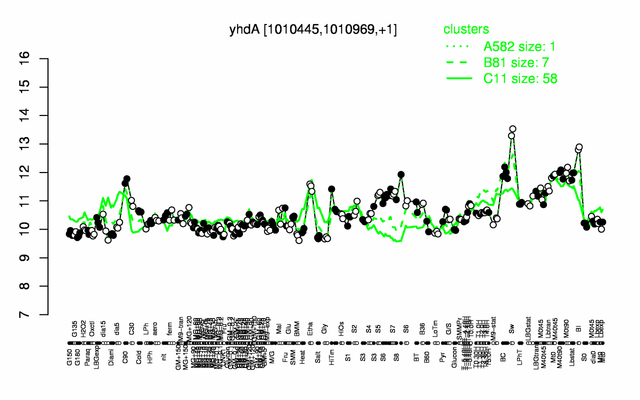
| |
Contents
Categories containing this gene/protein
poorly characterized/ putative enzymes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU09340
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: azoreductase type 2 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 2GSW
- UniProt: O07529
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
K Ramanathan, V Shanthi, Rao Sethumadhavan
In silico identification of catalytic residues in azobenzene reductase from Bacillus subtilis and its docking studies with azo dyes.
Interdiscip Sci: 2009, 1(4);290-7
[PubMed:20640807]
[WorldCat.org]
[DOI]
(P p)
Diana Wolf, Falk Kalamorz, Tina Wecke, Anna Juszczak, Ulrike Mäder, Georg Homuth, Sina Jordan, Janine Kirstein, Michael Hoppert, Birgit Voigt, Michael Hecker, Thorsten Mascher
In-depth profiling of the LiaR response of Bacillus subtilis.
J Bacteriol: 2010, 192(18);4680-93
[PubMed:20639339]
[WorldCat.org]
[DOI]
(I p)
Sina Jordan, Anja Junker, John D Helmann, Thorsten Mascher
Regulation of LiaRS-dependent gene expression in bacillus subtilis: identification of inhibitor proteins, regulator binding sites, and target genes of a conserved cell envelope stress-sensing two-component system.
J Bacteriol: 2006, 188(14);5153-66
[PubMed:16816187]
[WorldCat.org]
[DOI]
(P p)