Difference between revisions of "RnjA"
(→Original publications) |
(→Original publications) |
||
| Line 178: | Line 178: | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>25940620 18079181, 19553197, 17981983, 19458035, 15831787, 18204464, 18713320, 19193632, 17005971, 18445592, 17512403, 17229210, 17576666, 19210617 19633085 19638340 19850915 19880604 20025672 20418391 20572937 ,21803996 21862575 21925382 22198292 22412379 23504012 21893285,21893286,21908660 24187087</pubmed> | + | <pubmed>25940620 18079181, 19553197, 17981983, 19458035, 15831787, 18204464, 18713320, 19193632, 17005971, 18445592, 17512403, 17229210, 17576666, 19210617 19633085 19638340 19850915 19880604 20025672 20418391 20572937 ,21803996 21862575 21925382 22198292 22412379 23504012 21893285,21893286,21908660 24187087 26253740</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:13, 28 August 2015
- Description: RNase J1
| Gene name | rnjA |
| Synonyms | ykqC |
| Essential | yes PubMed |
| Product | RNase J1 |
| Function | RNA processing |
| Gene expression levels in SubtiExpress: rnjA | |
| Interactions involving this protein in SubtInteract: RNase J1 | |
| Metabolic function and regulation of this protein in SubtiPathways: rnjA | |
| MW, pI | 61 kDa, 5.902 |
| Gene length, protein length | 1665 bp, 555 aa |
| Immediate neighbours | adeC, rpoY |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed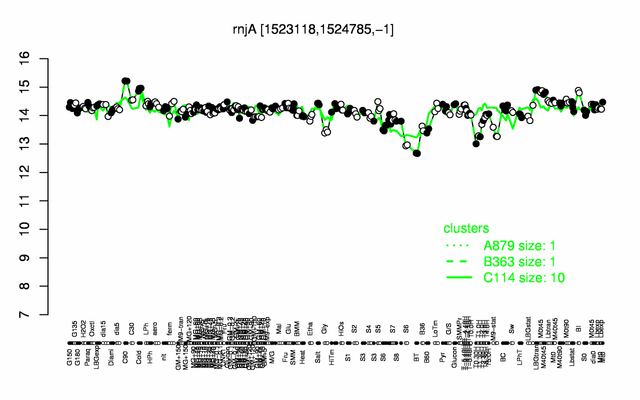
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU14530
Phenotypes of a mutant
- essential PubMed
- a study from the lab of Ciaran Condon reports that rnjA is non-essential and that the mutant is strongly impaired in sporulation, genetic competence and many other phenotypes PubMed
Database entries
- BsubCyc: BSU14530
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: endonuclease and 5'-3' exonuclease
- Protein family: RNase J subfamily (according to Swiss-Prot)
- Paralogous protein(s): RnjB
RNAs affected by rnjA
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (according to Swiss-Prot)
Database entries
- BsubCyc: BSU14530
- UniProt: Q45493
- KEGG entry: [2]
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
- required for thrS RNA processing, involved in maturation of the 5’-end of the16S rRNA
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
- translation of YkzG and RnjA is coupled, and this coupling is required for efficient expression of RNase J1 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 2868 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 4928 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 2768 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 4125 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 5056 PubMed
Biological materials
- Mutant:
- GP41 (rnjA under control of p(xyl)), available in Jörg Stülke's lab
- SSB342 (rnjA under pspac), cat, available in Harald Putzer lab
- Expression vector:
- for chromosomal expression of RNase J1-Strep (spc): GP1034, available in Jörg Stülke's lab
- for chromosomal expression of RNase J1-Strep (cat): GP1042, available in Jörg Stülke's lab
- lacZ fusion: pGP418 (in pAC7), available in Jörg Stülke's lab
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- FLAG-tag construct:
- GP1020 (spc, based on pGP1331), available in Jörg Stülke's lab
- GP1075 (aphA3), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Harald Putzer, IBPC Paris, France Homepage
David Bechhofer, Mount Sinai School, New York, USA Homepage
Ciaran Condon, IBPC, Paris, France Homepage
Your additional remarks
References
Reviews
Original publications