Difference between revisions of "AhpC"
| Line 120: | Line 120: | ||
** number of protein molecules per cell (minimal medium with glucose and ammonium): 24849 {{PubMed|24696501}} | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 24849 {{PubMed|24696501}} | ||
** number of protein molecules per cell (complex medium with amino acids, without glucose): 44043 {{PubMed|24696501}} | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 44043 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 43066 {{PubMed|21395229}} | ||
| + | |||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 62318 {{PubMed|21395229}} | ||
=Biological materials = | =Biological materials = | ||
| − | |||
* '''Mutant:''' | * '''Mutant:''' | ||
Revision as of 13:32, 17 April 2014
- Description: alkyl hydroperoxide reductase (small subunit)
| Gene name | ahpC |
| Synonyms | perR |
| Essential | no |
| Product | alkyl hydroperoxide reductase (small subunit) |
| Function | resistance against peroxide stres |
| Gene expression levels in SubtiExpress: ahpC | |
| Metabolic function and regulation of this protein in SubtiPathways: ahpC | |
| MW, pI | 20 kDa, 4.283 |
| Gene length, protein length | 561 bp, 187 aa |
| Immediate neighbours | gntZ, ahpF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 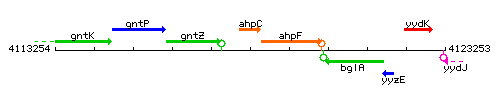
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
resistance against oxidative and electrophile stress, phosphoproteins, most abundant proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU40090
Phenotypes of a mutant
Database entries
- BsubCyc: BSU40090
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: 2 R'-SH + ROOH = R'-S-S-R' + H2O + ROH (according to Swiss-Prot)
- Protein family: ahpC/TSA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification: phosphorylated on ser/ thr/ tyr PubMed
- Effectors of protein activity:
Database entries
- BsubCyc: BSU40090
- UniProt: P80239
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
- belongs to the 100 most abundant proteins PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium): 24849 PubMed
- number of protein molecules per cell (complex medium with amino acids, without glucose): 44043 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 43066 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 62318 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Alain Lévine, Françoise Vannier, Cédric Absalon, Lauriane Kuhn, Peter Jackson, Elaine Scrivener, Valérie Labas, Joëlle Vinh, Patrick Courtney, Jérôme Garin, Simone J Séror
Analysis of the dynamic Bacillus subtilis Ser/Thr/Tyr phosphoproteome implicated in a wide variety of cellular processes.
Proteomics: 2006, 6(7);2157-73
[PubMed:16493705]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Annette Dreisbach, Dirk Albrecht, Jörg Bernhardt, Dörte Becher, Sandy Gentner, Le Thi Tam, Knut Büttner, Gerrit Buurman, Christian Scharf, Simone Venz, Uwe Völker, Michael Hecker
A comprehensive proteome map of growing Bacillus subtilis cells.
Proteomics: 2004, 4(10);2849-76
[PubMed:15378759]
[WorldCat.org]
[DOI]
(P p)
A F Herbig, J D Helmann
Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA.
Mol Microbiol: 2001, 41(4);849-59
[PubMed:11532148]
[WorldCat.org]
[DOI]
(P p)
N Bsat, L Chen, J D Helmann
Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes.
J Bacteriol: 1996, 178(22);6579-86
[PubMed:8932315]
[WorldCat.org]
[DOI]
(P p)
H Antelmann, S Engelmann, R Schmid, M Hecker
General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon.
J Bacteriol: 1996, 178(22);6571-8
[PubMed:8932314]
[WorldCat.org]
[DOI]
(P p)